INTRODUCTION
Dentistry is a field that involves the clinician and the patient being exposed to saliva and other infectious material. Prosthodontic patients are generally a high risk group relative to their potiential to transmit infectious diseases as well as acquire them. Therefore recently, an increase in the awareness of the need for cross-infection control measures to protect against possible routes of transmission has been discussed. In a country like India, high on disease incidence and sterilization protocol need to be implemented on the highest priority. The prosthodontists are at an added risk of transmission because of the infection spreading through the contaminated lab equipments while working in the lab. Therefore infection control is an important concept in the present day dentistry which has be followed in the clinics as well as in the laboratory. This article gives an overview of the possible risk of disease transmission and the ways to control them through effective sterilization and disinfection protocols.
Transmission of diseases
Micro-organisms capable of causing disease are present in human blood. Contact with blood or saliva mixed with blood may transmit pathogenic micro- organisms.1 The pathogens may be transmitted among the patient, dentist, dental care worker and the lab technician. Disease transfer to the dentist and dental staff during dental care is considered an “ occupational exposure” to a given pathogen on the other side the disease may also be transmitted from the dentist to the patient as well as from one patient to the other.2 The disease transfer from one patient to another in the dental clinics is considered as “ cross infection”. Infection control is aimed to prevent such type of transmissions in the dental office.3
The dental professional must assume that every patient treated is a risk of cross infection and to adopt appropriate control measure. The cycle of cross contamination is demonstrated in Figure 1.4
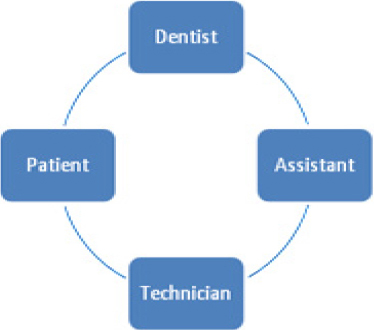
|
Figure 1: Cycle of cross contamination
Click here to view |
Organisms can be transmitted in dental settings through
1) Direct contact with blood, oral fluids, or other patient materials
2) Indirect contact with contaminated objects
3) Contact of conjuctival, nasal, or oral mucosa with droplets
4) Inhalation of airborne micro organisms that can remain suspended in the air for long periods.
Based on the article in DCNA 1996, 5 classes of risk have been described based on the level of risk and case of prevention.5

|
Click here to view |
Infection control
There are some procedures in infection control which are to be followed strictly to prevent any cross contamination. The main emphasis should not be only on patient protection but also on protection of the dental team.
The procedures involve
Patient screening
Personal hygiene
Personal protection
Instrument processing
Surface asepsis
Patient treatment
Laboratory disinfection
Patient screening
Any treatment has to be performed only after a comprehensive patient evaluation. This is generally accomplished by recording medical history specially designed to identify the patients who are either particularly susceptible to infection or who are at risk of transmitting infection, known as carriers of disease or by being in a high-risk category.6
Personal hygiene
Thorough forearm and hand washing is mandatory before and after treatment. Fingernails are kept clean and short to prevent perforation of gloves and accumulation of debris. Fingernail polish is not worn. Use surgical head cap, face mask and eye protecting glasses and long sleeved clinical coats.7
Personal protection
Residents are required to have current immunizations against communicable diseases including hepatitis B.3 The vaccination programme must certainly be considered the most effective cross infection control measure to protect dental personnel and in turn their patients from a potentially fatal disease.8 In june 1982, council on dental therapeutics adopted a resolution recommending that all dental personnel having patient contact including dentists, dental students and dental auxillary personnel , and all dental laboratory personnel receive the hepatitis B vaccine.9
Dental clinic personnel should wear appropriate protective barrier such as eye wear or chin length face shields, disposable gloves, disposable masks , protective garments, and other needed items when performing procedures capable of causing splash or spatter of blood or other potentially infectious materials.10
Disposable gloves are single-patient-use items. The type of the gloves is chosen based on the procedure being performed. Latex exam gloves are adequate for most dental procedures unless a sterile field requires the use of sterile gloves.10 The use of powder free gloves will minimize the risk of and alleviate the symptoms of latex allergy. If the dental care provider, dental staff or patient is allergic to latex, alternatives such as neoprene or nitrile gloves must be used. Utility gloves that are chemical and puncture resistant must be used when handling contaminated instruments, when performing operatory clean up, and for other contact with contaminated surfaces or disinfectant chemicals.
Masks are worn in the patient treatment area and when the dentist is manipulating the prostheses in the laboratory. The masks used should have at least 95% filtration rate of particles 3 to 5 microns in diameter.
Dental care providers and dental staff must wear protective eyeware which must be optically clear, antifog, distortion free , close fitting and shielded at the sides.
The level of protection from protective garments like long sleeves, closed neck, garment length, etc is determined by the procedure that is being performed. Generally cotton/polyester type fabric garments are also acceptable.
Instrument processing
-Presoaking and cleaning
-Packing
-Sterilization
Precleaning and cleaning solutions7

|
Click here to view |
Sterilizers

|
Click here to view |
Surface Asepsis:3
There are two general approaches to surface asepsis
-Clean and disinfect contaminated surface
-Prevent surface from becoming contaminated by use of surface covers
-A combination of both may also be used
According to Miller and Palenik in 1994 chemicals used for surface and equipment asepsis are
-Chlorine e.g. sodium hypochlorite
-Phenolic compounds —

|
Click here to view |
Patient Treatment
The responsibility for infection control procedures during patient treatment rests primarily on the dentist’s ability to adhere to strict sterilization, disinfection and barrier techniques. The use of strict system of zoning in the clinic will reduce the number of areas contaminated and there by maintain asepsis.
The working area is sprayed and left for 10 mins before any procedure starts along with the wiping of the operatory and chair with a disinfectant solution. The chair is covered with a disposable plastic sheath which has to be removed subsequent to the treatment. All the patients are advised to rinse with chlorhexidine gluconate 0.12% and wear protective eye wear before the commencement of the treatment.11
The operator should wash the hands with antimicrobial cleanser before gloving and should only touch the patient and the disinfected area once gloved. A unit dose concept may be adopted for use in the clinic as a cross-contamination control measure. This may be applied to many items and materials in prosthodontics, such as impression materials and waxes. The materials should be dispensed by a noncontaminated assistant prior to patient contact. This will eliminate the possibility of cross-contamination occurring via containers, tubes, and dispensers.
Large, non-sterilizable items used in the operatory, such as impression material dispensing guns, articulators, face bows, water bath, silicone spray bottles, tooth shade, and mold guides are disinfected by wiping, spraying, or immersion with the appropriate disinfectant solution.7
All items leaving the clinic that are used in direct patient care or touched during patient care procedures that cannot be subjected to sterilization procedures are disinfected or placed in the phenol disinfection solution within a sealed plastic bag before departure. New latex gloves are worn for the disinfection procedures.3
Disinfection of impressions
American Dental Association (ADA) guidelines state that impressions should be rinsed to remove saliva, blood and debris and then disinfected before being sent to the laboratory. When considering methods of disinfection for impressions, two factors are important: 1) the effect of the treatment on the dimensional stability and surface detail of the impression and 2) the deactivating effect of the impression material on the disinfecting solution, which could reduce the efficacy of the process.12
Polyvinylsiloxane, polysulfide, impression compound, and Zinc oxide eugenol (ZOE) impression materials are thoroughly rinsed under water and immersed in a 5.25% sodium hypochlorite solution for 10 minutes (9-12). Alginate and polyether impressions are rinsed under water, sprayed with a 5.25% sodium hypochlorite solution and sealed in a plastic bag for at least 10 minutes. 2% glutaraldehyde did not affect the accuracy and dimensional stability of polyether and polyvinyl siloxane impression materials after immersion for 30 or 60 minutes.13 Twenty minute immersion in 2% ID 210 solution (Durr Dental, Bissingen, Germany) has no adverse effects on the dimensional stability or surface detail reproduction of rigid material such as an impression compound, impression plaster, and zinc oxide-eugenol impression material.14
Impression trays

|
Click here to view |
Disinfection of wax bites/ rims, bite registrations
Wax, ZOE, and resin centric relation records are rinsed under water and sprayed with a 5.25% sodium hypochlorite solution and placed in a plastic bag for 10minutes.15
Disinfection of casts
Stone casts are the most difficult item to be disinfected without causing any much damage. Casts requiring disinfection are sprayed with a 5.25% sodium hypochlorite solution and allowed to sit for at least 10 minutes.3
Disinfection of dental prostheses
Complete dentures and provisional restorations that leave the operatory are immersed in a 5.25% sodium hypochlorite solution for 10 minutes. Removable partial dentures with metal bases are sprayed with 2% gluteraldehyde solution and held in a plastic bag for 10 minutes.3
Laboratory disinfection
The dental laboratory becomes the second line of infection control barriers that protect the patients, residents, assistants, and faculty. All prostheses that enter and leave the laboratory are disinfected. All prostheses entering the laboratory are scrubbed with disinfectant solution. Those leaving the laboratory are immersed in a 5.25% sodium hypochlorite solution for a minimum of 10 minutes. Laboratory countertops are cleaned and wiped with disinfectant solution at the end of each day. Individually packaged chemiclaved laboratory burs are available in the laboratory. After the desired procedure is accomplished, the laboratory bur is cleaned and placed in a new bag for sterilization. The burs are used for one patient only and then re sterilized. For polishing the lathe, when the technician should use individually packaged sterile polishing wheels, designated for use with pumice. The addition of an antiseptic product that contained Octenidine as active agent to conventional pumice reduced the number of microorganisms by 99.999%. The mix of steribim with water reduced the number of bacteria by 99%. The wheel is wet with water to soften it before use.16 If prosthesis becomes contaminated during laboratory procedures, it is disinfected and the laboratory procedure continued.
Final polish is accomplished using a sterile wheel with non contaminated acryluster. The acryluster is applied to the sterile wheel once before polishing to eliminate cross-contamination. Cleanup involves disposal of the plastic container and the contaminated pumice. Wheels are removed, rinsed under water, and bagged for autoclaving. Before returning to the main clinic, all items arc disinfected by immersion or spray and placed in a Lock-Tight bag. All information regarding disinfection procedures performed on Prosthodontic items sent to an outside laboratory is clearly written on the prescription form and the plastic bag. All items received from a laboratory are treated as contaminated unless the resident is informed otherwise by the dental laboratory.3
CONCLUSION
The increased awareness of the dangers of cross contamination with hepatitis B virus (HBV) and HIV during dental procedures is having a growing impact on attitudes toward infection control in the dental clinic and laboratory. Lack of Infection Control is life-threatening for both the patient and the Dental Professional and requires more efforts. Formal programs in Infection Control and Safety must be developed and strictly followed by the entire dental health care professional. Many Countries in the world have strong guidelines and recommendations for dental safety. In a country like India, the concept is new and needs to be advocated on the highest priority.
