Introduction
Periodontal diseases are a group of infectious diseases caused by predominantly Gram negative, anaerobic and microaerophillic bacteria that colonise the subgingival area resulting in inflammation of gingival and periodontal tissues and progressive loss of alveolar bone. Low birth weight (birth weight - <2.500 kilograms) is a major determinant of neonatal morbidity and mortality.1 Preterm low birth weight is recognized as a major cause of neonatal mortality and of nearly one- half of all serious long term neurological morbidity.2 Various primary and secondary risk indicators have been associated with the delivery of preterm low birth weight babies (Table 1).3 One of the major factors among these is infection, either sub-clinical or clinical. An association with maternal lower genitourinary tract infection, urinary tract-infection, cervical colonization with microbes etc. has been demonstrated by a number of studies.4, 5 Studies show that periodontitis may be a potential independent risk factor for preterm labor and / or low birth weight infants when all other known obstetric risk factors are not dominant and the pathogenic mechanism is postulated to be the same as with other maternal infection.6, 7 Inflamed periodontal tissues produce significant amount of proinflammatory cytokines, tumor necrosis factor-alfa (TNF-alfa) and prostaglandin-E2 (PG-E2) which stimulate labor and these may be responsible for PTLBW babies.8 These inflammatory cytokines may also cause placental tissue damage contributing to fetal growth restriction and low birth weight atbirth.9
|
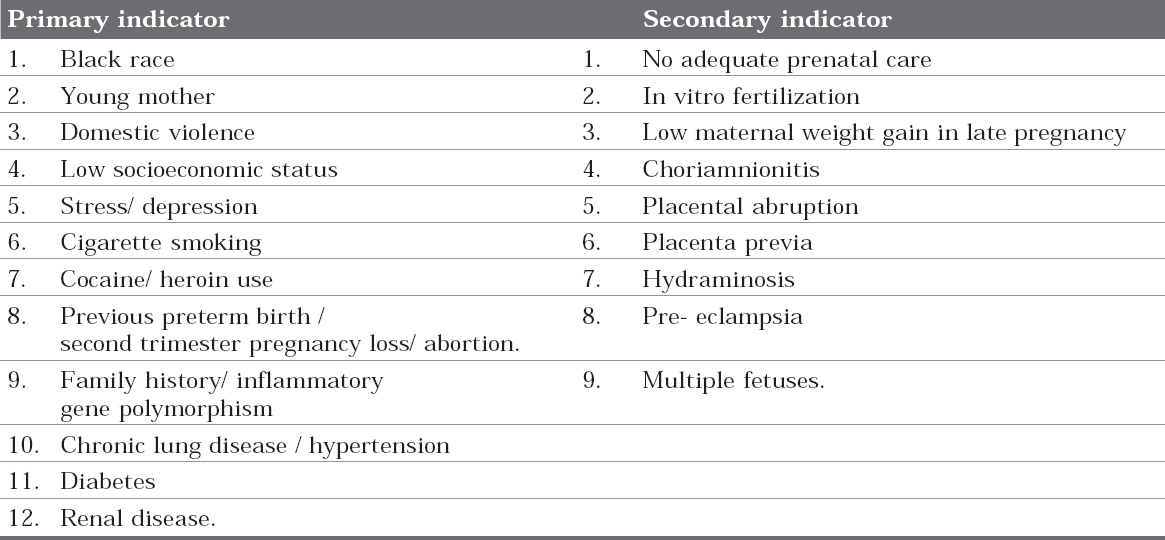
Table I: Risk Indicators of PTLBW3
Click here to view |
What is PTLBW?
The World Health Organization defines preterm birth as any live birth at less than 37 weeks of gestation. Delivery at less than 32 weeks is termed very preterm, and delivery at less than 28 weeks, as extremely preterm. The majority of preterm births are also low birth weight. The international definition of low birth weight adopted by the Twenty- ninth World Health Assembly in 1976 is a birth weight of “less than 2500 g’’.3
Can Infection Cause PTLBW
A large amount of evidence points to the role of infection as an etiologic factor for preterm birth. Repeatedly performed animal studies have demonstrated the capacity of administered bacteria or bacterial products to induce abortion. A substantial amount of data is available linking lower genital tract infection with preterm labour, premature rupture of membranes and low birth weight. Numerous reports indicate an association between bacterial vaginosis and preterm birth.10 The bacteria involved in chronic periodontal infection include gram-negative rods and anaerobes similar to those found in women with bacterial vaginosis. Both the above mentioned conditions, that are bacterial vaginosis and chronic periodontal infection, demonstrate a primary microbiological finding of quantitative overgrowth of anaerobic bacteria. Oral bacteria have the potential to lead to upper genital tract infection in pregnant women. As an example, Dixon et al reported a case of chorio—amnionitis at 24 weeks of gestation caused by Fusobacterium nucleatum and Capnocytophaga species.11 Fusobacterium species are common colonisers of the mouth, upper respiratory tract, and gastrointestinal tracts, but Capnocytophaga species are specifically oral commensals associated with periodontal infection. It has also been observed that tooth brushing is frequently associated with mild bacteremia. Therefore, it has been postulated that this bacteremia is followed by bacterial seeding of the placenta. In any case, it appears that the organisms that cause oral disease are similar to, if not identical to, those associated with upper genital tract infections, and that there is a plausible mechanism for the oral organisms to reach the placenta.
Ronald (2001) hypothesized the linking of subclinical infection and premature birth, as the microbes themselves or microbial toxins entering the uterine cavity during pregnancy by the ascending route from the lower genital tract or the blood borne route from a non-genital focus.12 Bacterial infection of the chorioamnion, or extraplacental membrane, may lead to chorioamnionitis, a condition strongly associated with premature membrane rupture and preterm delivery.13, 14 The biological mechanisms involve bacterially induced activation of cell-mediated immunity, which leads to production of cytokines (such as interleukins [IL-1 and IL-6] and tumour necrosis factor alpha [TNF-á]) and the ensuing synthesis and release of prostaglandins (especially prostaglandin E2 [PGE2]).13, 15 During normal pregnancy, the intra-amniotic levels of these mediators rise physiologically until a threshold level is reached, at which point labour, cervical dilatation and delivery are induced.3 Abnormal production of these mediators in the setting of infection triggers preterm labour and low birth weight.13, 15
However, many cases of histologically confirmed chorioamnionitis are not associated with active infection of the genitourinary tract and the results of culture are negative, both of which indicate that local infection is not the sole cause of this condition.13, 14 These findings led to speculation that an infection might be distant from the placental complex or the genitourinary tract and still present a risk for PLBW, as a result of the indirect action of translocated bacterial products such as endotoxins (specifically lipopolysaccharides [LPS]) or the action of maternally produced inflammatory mediators (or both).15
Association of Periodontitis and PTLBW
Periodontium, is the tissue surrounding and supporting a tooth and periodontal disease is a general term for a series of pathological alterations of the periodontium. When microorganisms are allowed to attach to the teeth, near the gum, usually what follows is inflammation of the gum (gingivitis). In this case, the small space between the gum and the teeth, named groove (normal), increases and, consequently, turns into a pocket (pathological). If the microbial flora of gingivitis is eliminated, the inflammation will recede and the gum will return to its normal status. If not properly treated, the pathological process of gingivitis may reach the hard tissue and slowly or abruptly cause alterations and result in periodontitis.6 The cause of these common inflammatory conditions is the dental plaque. In 1 mm3 of dental plaque weighing approximately 1 mg, more than 108 bacteria are present and over 300 species have been isolated and characterized in these deposits.16
The theory that remote sites of infection might contribute to PTLBW was supported by a number of studies using the pregnant golden hamster model.9, 17, 18 Pregnancy outcomes were evaluated in these animals after either the establishment of experimental periodontitis, the establishment of a non-disseminating subcutaneous tissue infection with Porphyromonas gingivalis (a common gram-negative periodontal pathogen)9 or intravenous injection of LPS from P. gingivalis.18,19 Fetal weights were significantly lower in the experimental animals, and the severity of the fetal effects was directly related to the levels of PGE2 and TNF-á. Drawing on the results of these animal studies, Offenbacher and his co-investigators developed a series of clinical studies to test the hypothesis that periodontal infections, serving as reservoirs for gram-negative bacteria, might pose a potential threat to the fetoplacental unit. In 1996, Offenbacheret al conducted a case control study in which they hypothesized that periodontal infections may have some kind of relationship with preterm births.20 They concluded that there was a statistical association between periodontitis in pregnant women, preterm births and low birth weight. Namely, they found that 18.2% of the incidence of preterm low birth weight could be attributed to periodontitis, making this an important risk factor not previously recognized.
In a subsequent case-control study, Offenbacher and others measured levels of PGE2 and IL-1 in the gingival crevicular fluid (GCF) of 48 mothers of PTLBW infants. In addition, the levels of 4 periodontal pathogens (Bacteroidesforsythus, P. gingivalis, Actinobacillus actinomycetem comitans and Treponema denticola) were measured with microbe-specific DNA probes.21 GCF levels of PGE2 were significantly higher in mothers of PTLBW infants than in mothers of infants with normal birth weight (controls). The 4 periodontal pathogens, characteristically associated with mature plaque and progressing periodontitis, were detected at significantly higher levels in the mothers of PTLBW infants. Furthermore, among the primiparous mothers of PTLBW infants, a significant inverse association was demonstrated between birth weight (as well as gestational age) and GCF PGE2, which suggests a dose-response relationship for increased GCF PGE2 as a marker of current periodontal disease activity and decreasing birth weight.21
More recently, Offenbacher’s group analyzed blood samples from fetal cords for the presence of immunoglobulin M (IgM) antibody against various periodontal pathogens. Of the PTLBW samples, 33.3% tested positive for IgM against the test bacteria, whereas only 17.9% of the normal birth weight samples tested positive. Of the 13 periodontal pathogens included in the analysis, IgM antibodies against Campylobacter rectus, P. gingivalis and Fusobacterium nucleatum were most often encountered. Although both preterm and normal birth weight infants had fetal cord IgM directed against specific bacteria, these fetal immune responses indicate that maternal periodontal infections can provide a systemic challenge to the fetus in utero.22
Also in other studies studies they found a statistically significant association between periodontitis and adverse pregnancy outcomes. 23-26 Although no definitive causal relationship has been established, and other explanations for the correlation might be offered, a model can nevertheless be envisaged wherein chronic periodontal infection could mediate this systemic effect through one or more of the following mechanisms:
Numerous studies show the prevalence and relationship between periodontal disease and PTLBW. Jeffcoat MKet al studied patients with severe or generalized periodontal disease had adjusted odds ratios (95 percent confidence interval[CI] of 4.45 (2.16-9.18) for preterm delivery.27 The adjusted odds ratio increased with increasing prematurity to 5.28 (2.05-13.60) before 35 weeks gestational age and to 7.07 (1.70-27.4) before 32 weeks gestational age. Mokeem SAet al found the prevalence of the PTLBW to be 11.3%, and the prevalence of periodontal disease was high among the study population.28 The risk of PTLBW remained high with increasing periodontal disease [OR 4.21, 95% CI (1.99-8.93)] despite controlling the other risk factors suchas age, smoking, and social class. Baskaradoss JK et al concluded that periodontitis was diagnosed in 25% of the mothers in the case group and in 14.5% of the mothers in the control group.29 Logistic regression analysis indicated a risk of nearly threefold for preterm delivery in mothers with periodontitis [adjusted odds ratio (OR) = 2.72; 95% confidence interval (CI): 1.68-6.84].
Conclusion
In this sense, it has been described that the oral condition of patients should not be considered as separate problems, but rather in relation to the human body as a whole therefore all periodontal infections must be recognized and treated, and then a regular oral care must be maintained. It has been well documented that periodontal disease is a treatable and preventable condition. Periodontal medicine promotes a strong collaboration between dental and medical professionals. In the event of a positive association of periodontal infection with PLBW, this would have potential applications in preventive oral health programs as an integral component of prenatal care for pregnant mothers. Women should be counseled on modifiable risk factors which will also encourage a healthier lifestyle. Indeed, as healthcare professionals working as a team, an understanding of the role of periodontal-systemic relationship and its implications will further enhance the quality of medical and dental care being provided to our patients in the community.
