Introduction
Fixed and removable prostheses are manufactured using metal alloys. It is not only important to know the physical and mechanical properties of these materials but also their biocompatibility and their resistance to corrosion. In the oral cavity, the saliva being the most corrosive agent, these structures are exposed to a chemically adverse environment.
The dental alloys are generally placed in the mouth for a correspondingly longer time, where they are subject to electrochemical reactions, mechanical forces of mastication, and generalized wear.1 These implants must not induce adverse biological reactions such as gingival swelling and erythema, mucosal pain and lichenoid reactions. Although these troubles are often caused not by the materials itself, they can be induced by the metallicions released during their corrosion.2 Through corrosion process, metal ions are released from dental alloys in oral cavity even though the protective oxide film exists on the metal surface.
Use of Gold in Dentistry
Gold has traditionally found use because of its good resistance to oxidative corrosion. Gold itself is considered chemically inert and biologically compatible with the body. If longevity, functionality, aesthetics, and biocompatibility, together with ease of manufacture are considered as the most important requirements, the optimum material for dental restorations is still a well-approved high gold alloy. Even at much higher expense, gold is used in dentistry because of its superior performance and aesthetic appeal. Gold alloys are used for fillings, crowns, bridges, and orthodontic appliances. Gold is used in dentistry because it is chemically inert, non allergenic, and easy for the dentist to work. Corrosion of noble alloys may be clinically visible if it is severe, but more often, the release of elements continues for months or years at low levels and is not visible to the eyes.3 Gold-based alloys are referred to as noble alloys, based upon their electrochemical properties. The corrosion resistance of the alloys is due to the high thermodynamic stability of the gold in the alloys.4
The oral cavity provides a harsh environment for the orthodontic appliance of any kind.5 Several types of corrosion can take place through 1 of 2 mechanisms. Metal ions can be deposited directly into the saliva, or the protective surface film on the metal can progressively dissolve.6 Corrosion of orthodontic appliances has been thoroughly studied.7-12 There are several consequences of corrosion in orthodontics.
Orthodontic wires are recommended by the dentists to regulate the arrangement of teeth. People having these orthodontic wires have to brush their teeth daily. The toothpaste that they use may corrode the orthodontic wires in the oral environment. Hence there is a need to investigate the influence of various toothpastes on the corrosion resistance of orthodontic wires made of many metals and alloys. The following work was undertaken to study the corrosion behaviour of 22ct gold in artificial saliva, in the absence and presence of a toothpaste sparkle fresh.
Materials and Methods
Materials
The metal specimen chosen for the present study was 22ct gold and the toothpaste was Sparkle Fresh. The composition of the toothpaste is given below:
Composition of Sparkle Fresh
Sodium Monofluorophosphate (Active Ingredients), Calcium carbonate, Carboxy methyl cellulose, Glycerine, Hydrated silica, Sodium benzoate, Sodium lauryl sulfate, Sodium saccharin, sorbitol, Tetra sodium pyrophosphate and water (Inactive ingredients).
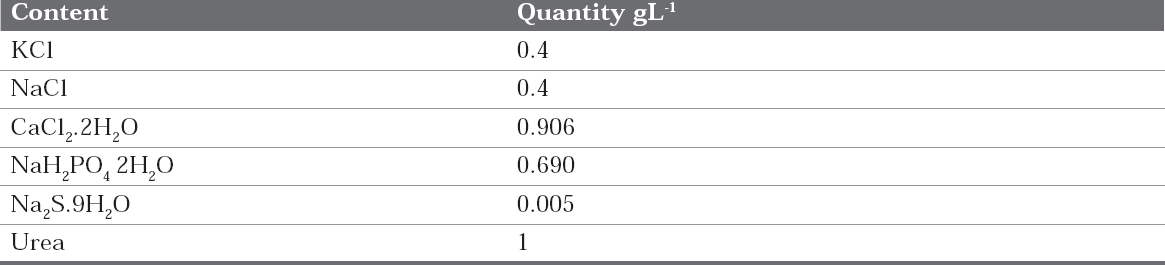
Fusayama Artificial Saliva
Fusayama was used as an electrolyte medium (Table 1).13 Fusayama artificial saliva solution constituents closely resemble those of natural saliva. During the study, the artificial saliva solution temperature was maintained at room temperature of 25%C.14
|
Table 1 : Chemical composition of artificial saliva (Fusayama Meyer)
Click here to view |
Methods
Potentiodynamic Polarization
Polarization studies were carried out in a CHI- Electrochemical workstation with impedance, Model 660A. A three electrode cell assembly was used. The working electrode was one of the metals. A saturated calomel electrode (SCE) was the reference electrode and platinum was the counter electrode. From the polarization study, corrosion parameters such as corrosion potential (E corr), corrosion current (Icorr), linear polarization resistance (LPR) and Tefel slopes (anodic =ba and cathodic=bc) were calculated.
AC Impedance Spectra
The instrument used for polarization study was used to record AC impedance spectra also. The cell set up was also the same. The real part (Z’) and imaginary part (Z”) of the cell impedance were measured in ohms at various frequencies. Values of the charge transfer resistance (Rt) and the double layer capacitance (Cdl) were calculated from Nyquist plots. Impedance log (Z/ohm) was calculated from Bode plots. During AC impedance spectra were recorded the scan rate (V/s) was 0.005; Hold time at Ef(s) was zero and quite time (s) was 2. The value of charge transfer resistance (Rt) and double layer capacitance (Cdl) were calculated from Nyquist plot.
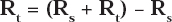
|
Click here to view |
Where Rs = Solution resistance, Rt =Charge transfer resistance
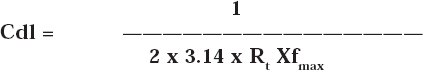
|
Click here to view |
Where fmax= frequency at maximum imaginary impedance.
Surface Characterization Studies
The thin wire metal specimen immersed in an inhibitor system, for a period of one day. The specimen was taken out, dried and the nature of the film formed on the surface of the metal specimen was analysed by surface analysis techniques.
Scanning Electron Microscopic Study (SEM)
The surface morphology was examined for the thin wire metal specimen in absence and in the presence of the inhibitor system by using Tescon, Vega3, and USA computer controlled scanning electron microscope. The specimen immersed in the system for a period of one day was removed, rinsed with double distilled water, dried and observed in a scanning electron microscope to examine the surface morphology.
Energy Dispersive Analysis of X-Rays (EDAX)
SEM imaging gives the morphological data for a sample; however by using x-ray spectroscopy in conjunction with SEM, the elemental composition can be determined. The elements present in a material are determined by an EDAX spectrum. An energy dispersive X-ray analyzer (EDAX) [Brucker, Nano, GMBH, Germany] unit attached to the SEM machine was used to carry out the elemental analysis of the metal surface.
Surface Analysis by FTIR Spectra
The FTIR spectra were recorded for the inhibitor and for the film formed on the thin wire metal specimen surface. A thin layer of inhibitor was applied on the metal surface, dried and was carefully scratched off. It was then mixed with KBr and made into pellets and the FTIR spectrum was recorded. The FTIR spectrum of film formed on the surface of thin wire metal specimen were recorded after immersion period of one day in artificial solution containing toothpaste Sparkle Fresh. The specimens were taken out of the test solutions and dried. The film formed on the surface was scratched carefully and it was thoroughly mixed with KBr, and made into pellets. FTIR spectrum of the powder (KBr pellet) was recorded using Perkin -Elmer 1600 FTIR spectrophotometer with a resolving power of 4 cm- 1.
UV- Visible Absorption Spectra
The possibility of the formation of metal - inhibitor complex in solution was examined by recording their UV-Visible absorption spectra for the blank, the inhibitor and the best system solution using Analytic Jena Specord S-100, UV -Visible spectrometer.
Fluorescence Spectroscopy
Fluorescence spectra of solutions, blank, the inhibitor and the best system were recorded by Using Jasco- 6300 spectroflurometer.
Results and Discussion
Analysis of Potentiodynamic Polarization Curves
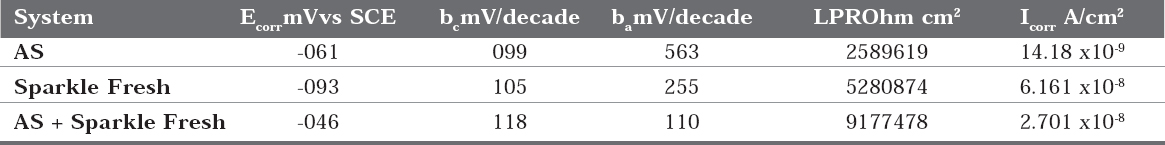
The corrosion parameters namely, corrosion potentiall (ECORR), Tafel slopes (b =cathodic; b = anodic), linear polarization resistance (LPR) and corrosion current (I corr) of 22ct gold immersed in artificial saliva (AS) in the absence and presence of tooth paste are given in Table 2 and the potentio dynamic polarization curves are shown in Figure 1.
|
Table 2 : Corrosion parameters of 22ct gold immersed in various test solutions obtained frompolarization study
Click here to view |
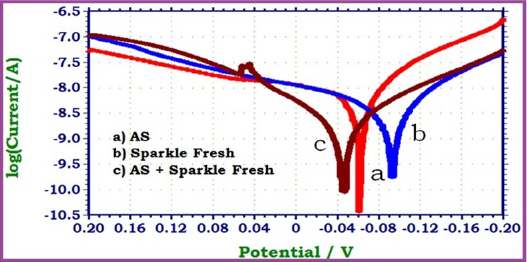
|
Fig 1 : Polarisation curves of 22 ct gold immersed in Artificial Saliva (AS) in the absence and presence of toothpaste Sparkle Fresh
Click here to view |
This observes that when 22ct gold immersed in AS, the corrosion potential is -061mV vs SCE. The linear polarization resistance value is 2589619 ohm cm2. The corrosion current is 14.18 x 10-9 A/cm2. When 22ct gold is immersed in aqueous solution of (1%) Sparkle Fresh, the corrosion potential is shifted to cathodic side (-093mV vs SCE). The linear polarization resistance (LPR) value increases from 2589619 ohm cm2 to 5280874 ohm cm2. The corrosion current decreases from 14.18 x 10 -9 A/cm2 to 6.161 x 10-9A/cm2. These observations indicate that the cathodic reaction is controlled predominantly. A protective film is formed on the metal surface. Hence linear polarization resistance (LPR) value increases and corrosion current (I corr) decreases. The protective film may probably consist of complexes formed between gold ion and the active principles of the ingredients of tooth pastes.
When 22ct gold is immersed in aqueous solutions consisting of AS and the 1% pastes solutions, the corrosion potential is shifted to the anodic side (-046 mV vs SCE). The shift is within 50 mV vs SCE. Hence it is inferred that, both anodic and cathodic reactions are controlled to an equal extent i.e. there is mixed type corrosion inhibition. Further, the linear polarization resistance (LPR) value increases from 2589619 ohm cm2 to 9177478 ohm cm2. Corrosion current decreases from 14.18 x 10-9 A/cm2 to 2.701 x 10-9. These observations indicate that the corrosion resistance of 22ct gold increases when it is immersed in AS containing aqueous solutions of the tooth paste Sparkle Fresh.
Analysis of AC Impedance Spectra
The AC impedance spectra of 22ct gold immersed in various test solutions are shown in fig 2, 3, 4 and 5. The Nyquist plots are shown in fig 2. The Bode plots are shown in figure 3, 4 and 5. The corrosion parameters derived from these plots are shown in table 3.
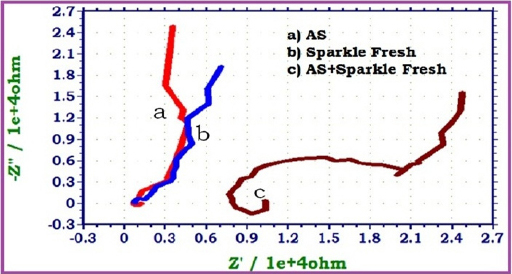
|
Fig 2 : AC impedance spectra of 22ct gold immersed in Artificial Saliva (AS) in the absence and presence of tooth paste Sparkle Fresh(Nyguist Plots)
Click here to view |
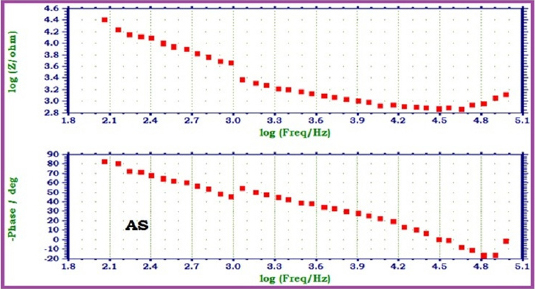
|
Fig 3 : AC impedance spectra of 22ct gold immersed in Artificial Saliva (AS) (Bode Plots)
Click here to view |
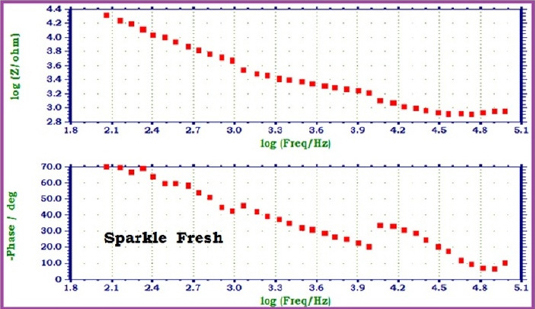
|
Fig 4 : AC impedance spectra of 22ct gold immersed in Sparkle Fresh (1%) (Bode Plots)
Click here to view |
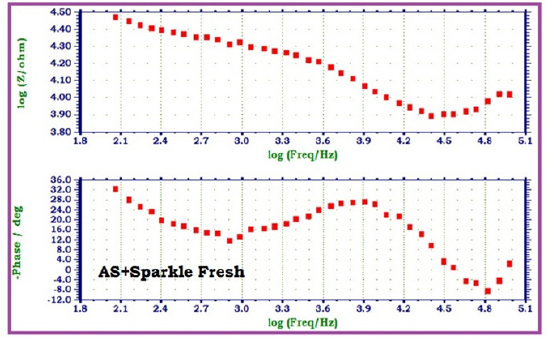
|
Fia 5 : AC impedance spectra of 22ct gold immersed in Artificial saliva (AS) in the presence of Sparkle Fresh (1%) (Bode Plots)
Click here to view |
|
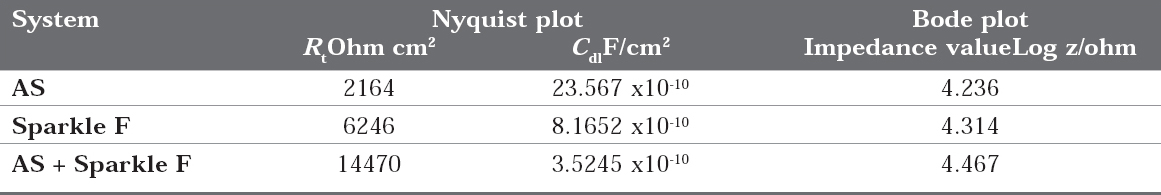
Table 3 : Corrosion parameters of 22ct gold immersed in various test solutions obtained from ACimpedance spectra.
Click here to view |
When 22ct gold is immersed in AS, the charge transfer resistance (Rt) value is 2164 ohm cm2, the double layer capacitance (Cdl) value is 23.567 x10-10 F/cm2 and the impedance (log z/ohm) value is 4.236. When 22ct gold is immersed in aqueous solutions of (1%) toothpaste Sparkle Fresh, the charge transfer resistance (Rt) value increases from 2164 ohm cm2 to 6246 ohm cm2, the double layer capacitance value (Cdl) value decreases from 23.567 x 10-10 to 8.1652 x 10-10 F/cm2 and the impedance value (log z/ohm) increases from 4.236 to 4.314. These observations indicate that, a protective film is formed on the metal surface when 22ct gold is immersed in aqueous solutions of tooth paste Sparkle Fresh. The protective film prevents the transfer of electrons from the metal surface to the bulk of the solutions. Hence corrosion resistance increases and the rate of corrosion decreases. The protective film probably consists of gold ion and the active principle of the ingredients of the tooth paste.
When 22ct gold is immersed in AS containing an aqueous solution of Sparkle Fresh, the charge transfer resistance (Rt) value increases from 2164 ohm cm2 to 14470 ohm cm2, the double layer capacitance (Cdl) value decreases from 23.567 x 1010 to 3.5245 x 10-10, the impedance (logz/ohm) value increases from 4.236 to 4.467. It inferred that, in presence of AS containing Sparkle Fresh, the corrosion resistance of 22ct gold increases.
SEM Analysis of Metal Surface
The SEM images for 22ct gold in the absence and in the presence of the inhibitor system is shown in Figure 6 (a and b). Surface is found to be very smooth only for pure polished 22ct gold. But for the inhibitor system, surface has become rough due to the presence of film deposited on the metal surface. This protective film is due to the deposition of active principles of the ingredients present in the toothpaste. This protective film prevents the corrosion of 22ct gold in presence of artificial saliva and the toothpaste.
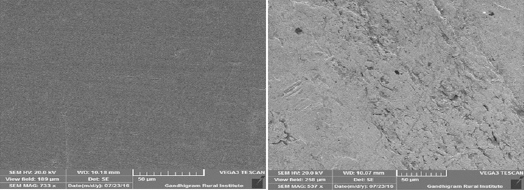
|
Fig 6 : SEM images for (a) Polished 22ct gold (b) Polished 22ct gold immersed in AS containing sparkle fresh toothpaste Click here to view |
Energy Dispersive Analysis of X -Rays (EDAX)
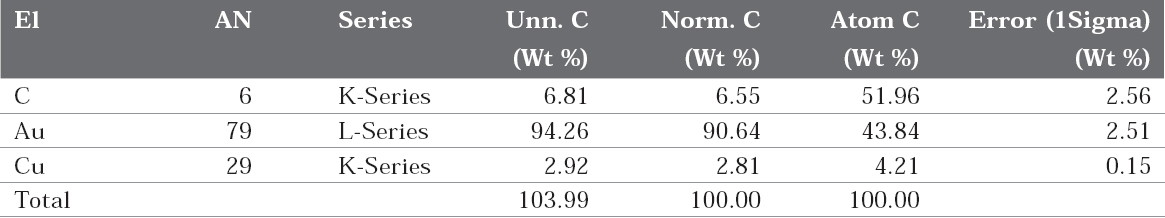
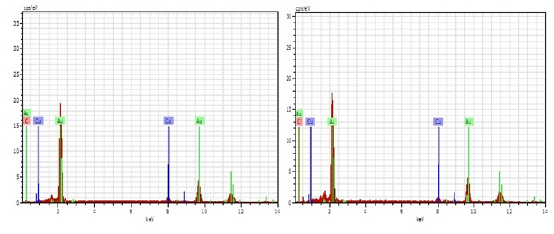
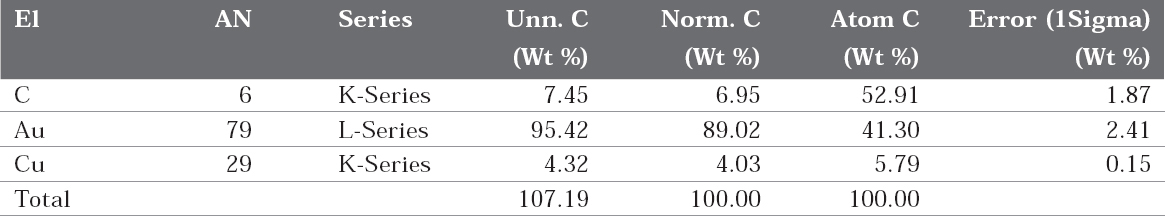
The EDAX spectra are shown in figure 7 (a and b). It is seen from the EDAX spectra that Au and Cu are present in both absence and presence of the inhibitor (Table 4 and 5). But the weight percentage of gold and copper has changed after immersion in the artificial saliva containing sparkle fresh toothpaste.
|
Fig 7 : EDAX spectra of (a) Polished 22ct gold (b) Polished 22ct gold immersed in AS containing sparkle fresh toothpaste Click here to view |
|
Table 4 : Spectrum of 22ct gold
Click here to view |
|
Table 5 : Spectrum of 22 ct gold +AS+ Sparkle fresh toothpaste
Click here to view |
In the case of bare 22ct gold, because of the presence of the copper, the metal would have undergone atmospheric corrosion due to leaching out of copper and copper oxide would have formed on the metal surface. This layer would have decreased the intensity of gold (Figure 7b).
When the electrode is immersed in the environment consisting of saliva and the toothpaste, the active principles of the ingredients of the toothpaste would have formed a protective film on the metal surface, thus preventing the corrosion of 22ct gold. This results in the increase in the intensity of gold. The increase in corrosion resistance is supported by electrochemical studies.
Analysis of FTIR Spectra
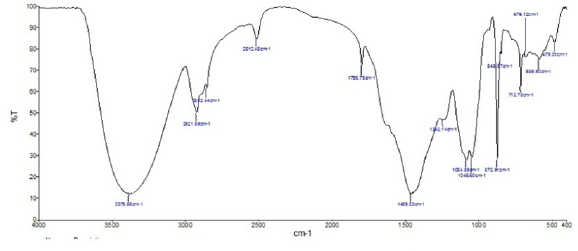
The FTIR (KBr) spectrum of pure toothpaste sparkle fresh is shown in figure 8. Analysis of the structures of these compounds reveals that the active principles of the ingredients of toothpaste sparkle fresh contain functional groups like OH, C=O, S, N and an aromatic ring. The peak appears at 3379cm-1 is due to O-H stretching frequency. 2921.59 cm-1 and 2852.44cm-1peaks are due to C-H stretching frequency. S-H stretching frequency was observed at 2512.45cm-1. 1796.75 cm-1 peak is due to C=O stretching and 1084 cm-1 and 1046 cm-1 peaks are due to P-O stretching. 1242.14 cm-1peak is of P=O stretching and 1084 cm-1 peak is of C-N stretching. Peak at 1459.83 cm-1 indicate the presence of aromatic ring in the compounds present in the toothpaste.
|
Fig 8 : The FTIR (KBr) spectrum of pure toothpaste sparkle fresh.
Click here to view |
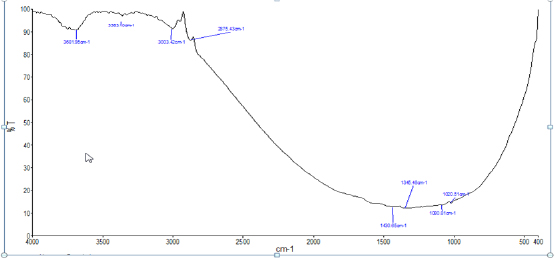
The FTIR spectrum of the film formed on the metal surface after immersion in artificial saliva containing toothpaste sparkle fresh is shown in figure 9. A shift is observed in the peak due to O-H stretching frequency from 3379 cm-1 to 3363.10 cm- 1. Peak due to C-H stretching frequency has shifted from 2852.44 cm-1 to 2875.43cm-1.. The peaks at 1796.75 cm-1 for C=O stretching frequency, 2512.45 cm-1 for S-H stretching frequency and 1242.14 cm- 1peak for P = O stretching frequency have disappeared as sulphur atom of S-H group and oxygen atom of C=O and PO groups having lone pair of electrons present in toothpaste sparkle fresh has co-ordinated strongly to gold ion and formed a complex. Peaks due to P-O stretching frequency have shifted from 1084 cm-1 to 1080.81 cm-1 and 1046 cm- 1 to 1020.51 cm-1. Peak for C-N stretching has shifted from 1084 cm-1 to 1080.81 cm-1. A shift was also observed in frequency due to aromatic ring from 1459.83 cm-1 to 1430.65 cm-1.
|
Fig 9 : The spectrum of the film formed on the metal surface after immersion in artifical saliva containing toothpaste sparkle fresh
Click here to view |
This result suggests that the active principles present in ingredients of toothpaste sparkle fresh have coordinated with the metal through oxygen atom of OH group, C=O group and PO group and sulphur atom of S-H group and nitrogen atom of C-N group forming a protective film which prevents the corrosion of 22ct gold. Compounds containing nitrogen, oxygen and sulphur can provide excellent corrosion protection efficiency compared with compounds containing either nitrogen or sulphur or oxygen.15 Synergistic effect of corrosion inhibition is observed.
Analysis of UV- Visible Absorption Spectra of Solutions
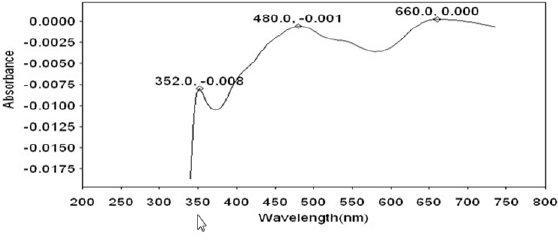
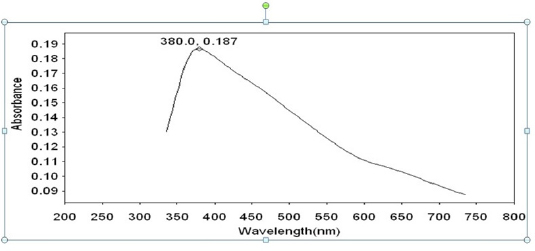
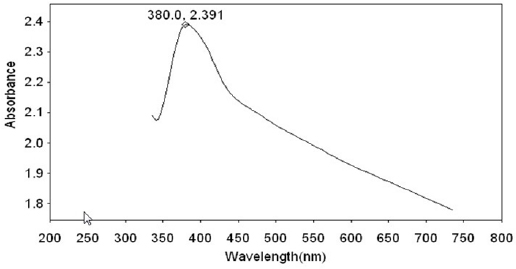
The UV- visible absorption spectrum is used to confirm the protective film formed on the metal surface. The UV- visible absorption spectrum of artificial saliva is shown in Figure 10. Peaks appear at 352 nm, 480 nm and 660 nm. The UV- visible absorption spectrum of toothpaste solution is shown in Figure 11. A peak appears at 380 nm. The UV- visible absorption spectrum of the solution of AS toothpaste system wherein 22 ct gold has been immersed for one day is shown in Figure 12. A peak appears at 380 nm. There is no shift in the position of ë max of the toothpaste system. This indicates that gold has not undergone corrosion in presence of saliva and toothpaste system. Had there been corrosion, there would have been shift in the position of ë max. The increase in intensity at 380 nm may be attributed to the fact that there is no electronic transition because of co-ordination of the active principles of the ingredients of the toothpaste with 22ct gold.
|
Fig 10 : UV-Visible absorption spectrum of artifical saliva
Click here to view |
|
Fig 11 : UV-Visible absorption spectrum of solution coutaining Sparkle fresh toothpaste.
Click here to view |
|
Fig 12 : UV-Visible absorption spectrum of solution coutaining 22ct gold + AS+ Sparkle fresh toothpaste
Click here to view |
Analysis of Fluorescence Spectroscopy
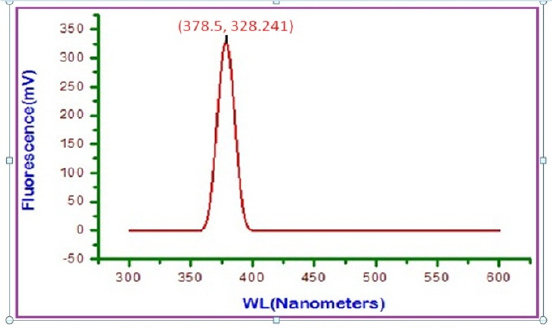
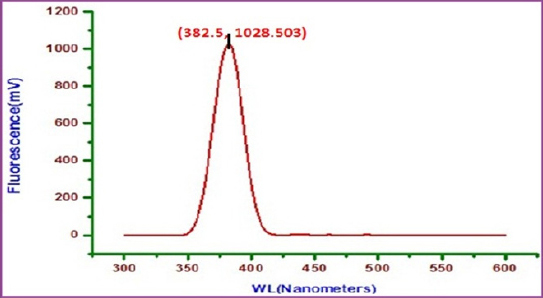
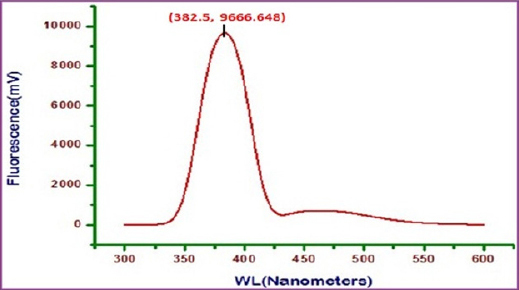
Fluorescence spectrum is used to detect the presence of metal- inhibitor complex formed on the surface of 22 ct gold alloy. The fluorescence spectrum (ëex= 300 nm) of artificial saliva is shown in figure 13. A peak appears at 378.5 nm. The fluorescence spectrum (ë =300 nm.) of an aqueous solution of sparkle fresh toothpaste is shown in figure 14. Emission takes place at 381.5 nm. 22ct gold was immersed in an aqueous solution containing artificial saliva and the toothpaste sparkle fresh. A solution was obtained. The fluorescence spectrum (ëex= 300 nm)of this solution is shown in figure 15. A peak appears at 385.5 nm. The shift in ë is not substantial. It is very close to that of toothpaste only.
|
Fig 13 : Fluorescence spectrum of artificial saliya (AS) solution
Click here to view |
|
Fig 14 : Fluorescence spectrum of solution containing Sparkle Fresh toothpaste
Click here to view |
|
Fig 15 : Fluorescence spectrum of solution containing 22ct gold + AS+ Sparkle Fresh toothpaste
Click here to view |
This indicates that 22ctgold has not undergone substantial corrosion in presence of AS and toothpaste. However a small hump (shoulder) appears around 460 nm. This may be due to the release of some Cuions in this system. This indicates there is slight corrosion of 22ct gold in this medium.
Conclusion
The corrosion resistance of 22ct gold in artificial saliva in the absence and the presence of toothpaste Sparkle Fresh has been evaluated by electrochemical studies such as polarization and AC impedance spectra and surface characterization studies such as SEM, EDAX, FTIR, UV-Visible absorption and fluorescence spectra. It is observed that the corrosion resistance of 22ct gold is more in the presence of toothpaste than in the presence of artificial saliva only. In the presence of artificial saliva and toothpaste the corrosion resistance still increases. This is due to the fact that the active principles of the ingredients of the toothpaste would have coordinated with the metal ion through their polar groups such as oxygen, nitrogen and sulphur forming a protective layer on the surface of the metal. The corrosion inhibition is enhanced due to the combinatorial approach of the compounds present in the toothpaste thus exhibiting the synergistic effect. The corrosion resistance increases in the order: AS+toothpaste > toothpaste > AS. The implication of this study is that people who have been implanted with orthodontic wires made of 18ct gold need not hesitate to clean their teeth with Sparkle Fresh toothpaste
