INTRODUCTION
The innate immune system is critical for the defense against pathogenic microorganisms. The host faces major challenges from microbial pathogens escaped from the host immune system. The innate immune system which act as first line of defense, relies on the presence of pattern recognition receptors, including membrane-bound Toll-like receptors, retinoid- inducible gene 1-like receptors, C-type lectin receptors and nucleotide-binding- domain-like receptors, to recognize various pathogens and their components.1, 2 These special receptors are expressed by macrophages, neutrophils, monocytes and epithelial cells. They respond to ’pathogen-associated molecular patterns (PAMP)’ and ’danger-associated molecular patterns (DAMP). DAMPs are of host derived (ATP, DNA or cholesterol crystals) or environmentally (asbestos, silica) derived.3 The activation of pattern recognition receptors by PAMPs and DAMPs leads to activation of specific inflammatory response by ’inflammasome’ complex.
Inflammasome: The term ’inflammasome’ was coined by Jurg Tschopp and his research team in 2002. Inflammasome complex is nucleotide-binding- domain-like receptors containing multi protein complexes. They are activated by exposure to cellular danger or stress signals, which trigger release of pro-inflammatory cytokines, such as interleukin-1ß (IL-1) and interleukin-18(IL-18).4 Nucleotide-binding-domain-like receptors (NLRs) are a family of intracellular immune receptors which consists of leucine-rich repeats (LRRs) near the C terminus and a nucleotide-binding domain (NBD). The LRR domain plays role in auto regulation, the recognition of PAMPs and protein-protein interactions. The NBDs regulates self- oligomerization.5, 6
NLRs differ in their N-terminal domains.7 The largest group contains 14 members which have N- terminal pyrin domain (PYD). Another group contains an N-terminal caspase recruitment domain (CARD), and other group contains nucleotide- binding oligomerization domains (NOD) Other NLR family members contains an acidic transactivation domains. Several members of the NLR family assemble as multi molecular complexes in response to various activators, leading to the activation of inflammatory caspases. Activated caspase-1 controls the maturation of the cytokines of the IL-1 family. These NLRs complexes are called inflammasome. The inflammasome are NLRP1, NLRP3, NLRC4 and AIM2 (Figure 1) which belongs to a different protein family (PYHIN).8-11
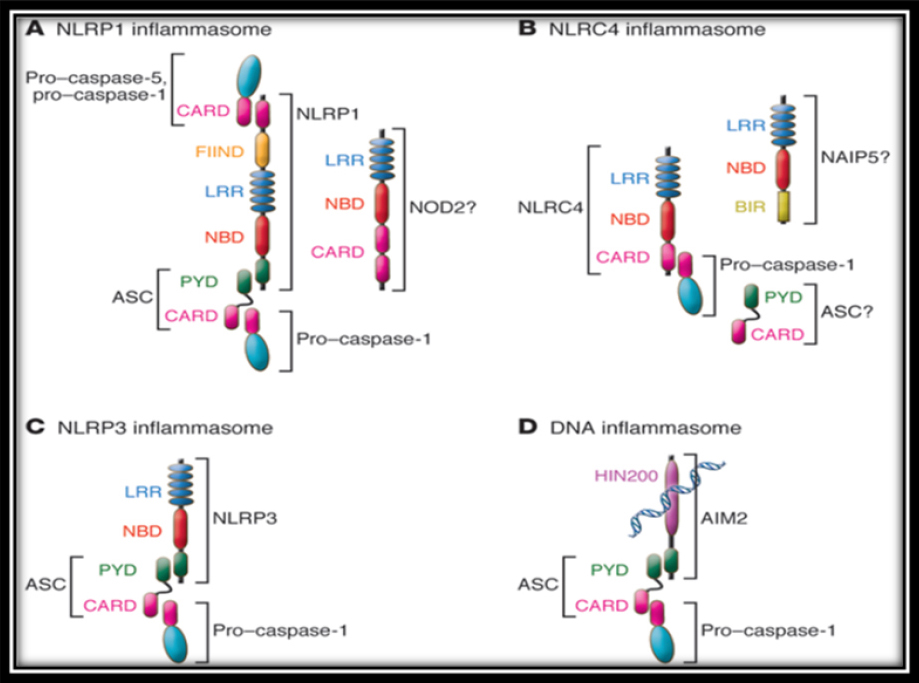
|
Figure 1: Nucleotide-binding-domain-like receptors (NLRs)
Click here to view |
Inflammasome can control the mediation of proinflammatory responses in a group of chronic diseases, such as gout, cancer and bacterial and viral infections.2, 12 The dysregulation of inflammasome components are associated with various inherited chronic inflammatory and immune disorders.3 In particular, the imbalance of interleukin-1ß activity is among the focal points of both microbial- associated and non microbial inflammatory diseases. The progression of periodontitis is inflammatory in nature, with the main triggers of oral inflammation usually residing in the oral micro biome and the balance of its components.13
Activation of NLRP3 inflammasome
NLRP3 is one of the best characterized nucleotide- binding-domain-like receptor family which plays a key role in the induction of pro-inflammatory host responses.14 Microbial infection individual microbial components and host-derived small danger molecules, such as extracellular ATP activates NLRP3. 15-17 The stimulated NLRP3 binds to caspase-1, leads to the activation of caspase-1, which ultimately cleaves pro- interleukin-1ß and pro-interleukin-18 into their biologically active mature forms.4
NLRP3 inflammasome activation generally requires two signals (Figure 2):
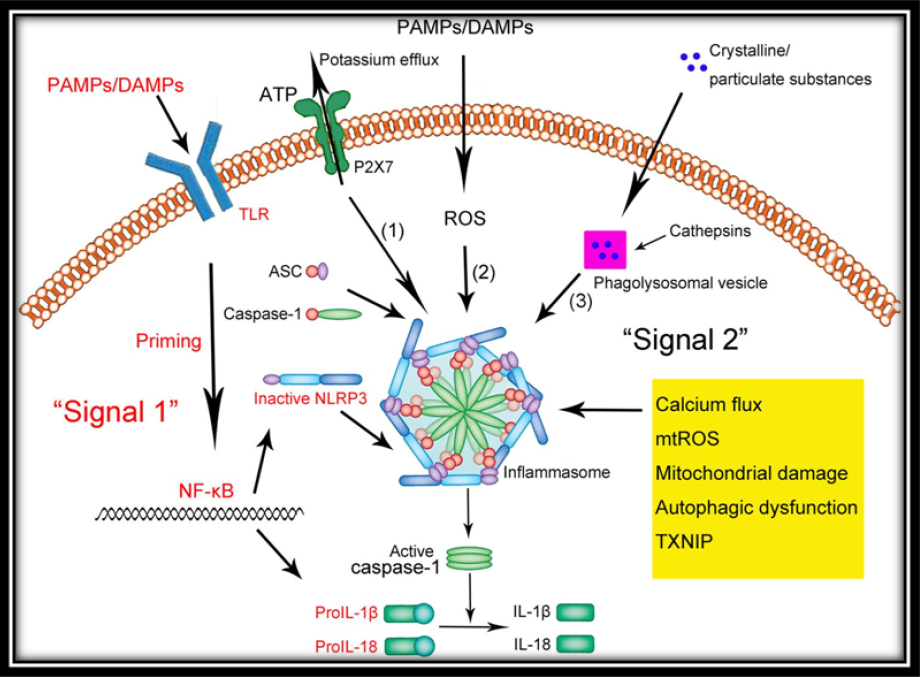
|
Figure 2: Inflammasome Signaling Pathway
Click here to view |
I. The first signal is induced when PAMPs stimulate a pattern- recognition receptor and leads to production of the interleukin-1 precursor.18
II. The second signal is induced by DAMPs
A growing number of research findings are highlighting the crucial role of extracellular ATP in the regulation of the NLRP3 inflammasome through purinergic receptors (P2X).19 The significance of the P2X7 receptor is widely studied in myeloid cells, such as monocytes, macrophages and dentritic cells. The role of P2X7 in epithelial cells is recently explored. Gingival epithelial cells which are first line of innate immunity, express functional P2X7 receptors.20, 21 Recent studies have shown the role of P2X7 in the production of intracellular reactive oxygen species (ROS), which in turn leads to the activation of the NLRP3 inflammasome.22, 23
Potential mechanisms of NLRP3 inflammasome activation
Currently, there are three models for activation of the NLRP3 inflammasome:
1) The reactive oxygen species (ROS) model
2) The lysosomal burst model
3) The ATP-triggered K+-efflux model
Inflammasome signaling and periodontal disease
Inflammasome complexes play a pivotal role in periodontal disease and the inflammasome- associated inflammatory mediators involved in the progression of the disease have been highlighted through several clinical studies. 24-26 The relationship between the IL-1 cytokine family and the NLRP3 inflammasome complex has been studied recently.24 The findings of this study showed higher levels of NLRP3, NLRP2, IL-1 β and IL-18 mRNA in gingival tissue samples from patients with periodontal disease and also showed a positive correlation between NLRP3 and expression of IL-1 β and IL-18 in periodontal disease.24 Certain species of periodontal bacteria, such as P. gingivalis(pg), Treponema denticola(td), Tannerella forsythia (tf) and Eubacterium nodatum, were showed correlation with IL-1 β and IL-18 in GCF who suffered with chronic periodontitis.26
Key players in periodontal disease and inflammasome signaling
Porphyromonas gingivalis (P. g)
Porphyromonas gingivalis is a gram-negative host-adapted anaerobe and a prominent bacterium present in the tissues of patients with chronic severe periodontitis.21, 27, 28 Pg plays a key role in both oral and systemic diseases.13, 29 It showed distinct mechanisms for manipulating host inflammatory responses, such as reducing the innate immune response for its own benefit and, simultaneously, providing a favorable environment for co-habitants, such as F. nucleatum and T. denticola.30 It showed significant down-regulation of the expression of NLRP3 inflammasome and IL-1ß was seen in periodontal tissues when P. gingivalis was introduced in a subgingival biofilm.31 But in macrophages, NLRP3 inflammasome was up regulated in the presence of P. gingivalis and leads to increased levels of IL-1 β which results highly inflammatory cell death known as ’pyroptosis’. P. gingivalis can inhibit the extracellular ATP- P2X7 pathway by directly preventing inflammasome activation through secreting an effector called ’nucleoside diphosphate kinase’.20 Silencing of pannexin-1 expression in gingival epithelial cells resulted in the inhibition of extracellular ATP release during P. gingivalis infection, highlighting the importance of pannexin-1 in activation of the inflammasome and secretion of interleukin-1 β.20
Aggregatibacter actinomycetemcomitans (Aa)
Aggregatibacter actinomycetemcomitans has been closely associated with the loss of periodontal tissue attachment in affected sites of both adults and juveniles. The presence of A. actinomycetemcomitans is a well-identified indicator of the initiation of localized aggressive periodontitis.32 The pathogenicity of the microorganism is also underlined by its well- characterized virulence factors, such as leukotoxin and cytolethal distending toxin, which are suggested to play important roles in altering host inflammatory responses as well as in contributing to periodontal disease progression.33
A. actinomycetemcomitans up regulates NLRP3, IL 1β and down regulates NLRP6 in peripheral mononuclear leukocytes. The bacterial leukotoxin induced an excessive proinflammatory response in macrophages, through the secretion of IL-1 β and IL-18 and the involvement of purinergic receptor P2X7 in the process.34 In contrast, in another in vitro study leukotoxin and cytolethal distending toxin gene knockout mutant strains of A. actinomycetemcomitans were used to infect human mononuclear leukocytes, only up-regulation of NLRP3, interleukin- 1β, interleukin-18 and reduction of NLRP6 were observed.31 Based on the results of this study, there was possible regulation of inflammasome complexes by additional molecules other than the two best-studied virulence factors of the microorganism. A potential candidate may be ’bacterial IL-1 β receptor I’, a putative membrane protein of A. actinomycetemcomitans, which was found to bind IL-1 β.35
Although ’bacterial IL-1 β receptor I’ role is not identified clearly. There is a need for future studies addressing the role of host immune signaling cascades which are involved in the virulence of this pathogen.
Candida albicans
Candida albicans is an opportunistic fungal pathogen that commonly resides on human mucosal surfaces and, when overgrown under immuno compromised conditions, causes inflammation and other systemic infections.36 One of the most characterized virulence-associated factors belongs to the family of the ’secretion of aspartic proteases’, which has been shown to induce secretion of pro- inflammatory cytokines in human monocytes.37
A recent in vitro discovery demonstrated that secretion of asparticproteases-2 and -6 specifically were responsible for inducing interleukin-1beta and interleukin-18 production in human monocytes as a result of activation of NLRP3 inflammasome and caspase-1.
Another study showed that both NLRC4 and NLRP3 inflammasomes were important in the induction of secretion IL-1 β. These studies demonstrate the ability of C. albicans to induce excessive inflammatory responses.38
CONCLUSION
Periodontal disease is a chronic inflammatory disease. Innate immune system in periodontal disease plays a vital role in arresting of the disease. Besides other components of innate immune system, the inflammasome play a key role in the arrest of periodontal disease by activation of pro inflammatory cytokines and regulation of immune system.
