|
|
| Line 6: |
Line 6: |
| | <head> | | <head> |
| | <meta http-equiv="Content-Type" content="text/html; charset=UTF-8"> | | <meta http-equiv="Content-Type" content="text/html; charset=UTF-8"> |
| − | <title>Velija-Asimi, Burekovic, Dujic, Dizdarevic-Bostandzic, and Semiz: </title> | + | <title>Venkatesh V, Srinivas Gadipelly, Pavan Kumar, Brahmaji Rao, Haripriya Chari: </title> |
| − | <link rel = "stylesheet" | + | <link rel="stylesheet" type="text/css" href="http://nacd.in/ijda/css/style.css" media="screen" /> |
| − | type = "text/css"
| |
| − | href = "http://nacd.in/docs/html_style_sheet.css" />
| |
| | </meta> | | </meta> |
| | </head> | | </head> |
| | <body> | | <body> |
| | <div class="front" id="article-front"> | | <div class="front" id="article-front"> |
| − | <font face="Tahoma" color="black" size="2"> | + | <font face="Arial" color="black" size="2"> |
| | <h1 class="document-title">Bilateral Sinus Lift Procedure With Platelet Rich Fibrin (PRF) Alone And Demineralized Freeze Dried Bone Allograft (DFDBA) Material With Bio-Membrane - A Comparative Study</h1> | | <h1 class="document-title">Bilateral Sinus Lift Procedure With Platelet Rich Fibrin (PRF) Alone And Demineralized Freeze Dried Bone Allograft (DFDBA) Material With Bio-Membrane - A Comparative Study</h1> |
| | <h2 class="affiliation-title">Venkatesh V<sup><a class="sup-aff" href="#aff1">1</a></sup>, Srinivas Gadipelly<sup><a class="sup-aff" href="#aff2">2</a></sup>, Pavan Kumar<sup><a class="sup-aff" href="#aff3">3</a></sup>, Brahmaji Rao<sup><a class="sup-aff" href="#aff4">4</a></sup>, Haripriya Chari<sup><a class="sup-aff" href="#aff5">5</a></sup></h2> | | <h2 class="affiliation-title">Venkatesh V<sup><a class="sup-aff" href="#aff1">1</a></sup>, Srinivas Gadipelly<sup><a class="sup-aff" href="#aff2">2</a></sup>, Pavan Kumar<sup><a class="sup-aff" href="#aff3">3</a></sup>, Brahmaji Rao<sup><a class="sup-aff" href="#aff4">4</a></sup>, Haripriya Chari<sup><a class="sup-aff" href="#aff5">5</a></sup></h2> |
| Line 24: |
Line 22: |
| | <div>doi: 10.5866/2016.8.10207</div> | | <div>doi: 10.5866/2016.8.10207</div> |
| | <hr class="part-rule"/> | | <hr class="part-rule"/> |
| − | <p class="callout-title"><span class="generated">ABSTRACT</span></p> | + | <p class="callout-title"><span class="generated"><b>ABSTRACT</b></span></p> |
| − | <p style="text-indent:0pt; text-align:justify; margin-top:0.5em; margin-bottom:0.5em;"><span><b>Context:</b></span> Anatomic limitations often associated with the posterior maxilla are a flat palatal vault, deficient alveolar height, inadequate posterior alveolus, increased pneumatization of the maxillary sinus and close approximation of the sinus to crestal bone. The present study was taken to evaluate the alveolar bone growth on maxillary sinus after bilateral sinus lift procedures, performed using platelet-rich fibrin (PRF) alone on one side and Demineralized freeze dried bone allograft (DFDBA) with bio membrane on the other</p> | + | <p style="text-indent:0pt; text-align:justify; margin-right: 2em; margin-left: 1.5em; margin-top:0.5em; margin-bottom:0.5em;"><span><b>Context:</b></span> Anatomic limitations often associated with the posterior maxilla are a flat palatal vault, deficient alveolar height, inadequate posterior alveolus, increased pneumatization of the maxillary sinus and close approximation of the sinus to crestal bone. The present study was taken to evaluate the alveolar bone growth on maxillary sinus after bilateral sinus lift procedures, performed using platelet-rich fibrin (PRF) alone on one side and Demineralized freeze dried bone allograft (DFDBA) with bio membrane on the other</p> |
| − | <p style="text-indent:0pt; text-align:justify; margin-top:0.5em; margin-bottom:0.5em;"><span><b>Aims and objectives:</b></span> The aim and objective of this study is to compare and evaluate the alveolar bone growth on maxillary sinus after bilateral sinus lift procedures, performed using PRF alone on one side and DFDBA with bio membrane on the other with the simultaneous placement of dental implants.</p> | + | <p style="text-indent:0pt; text-align:justify; margin-right: 2em; margin-left: 1.5em; margin-top:0.5em; margin-bottom:0.5em;"><span><b>Aims and objectives:</b></span> The aim and objective of this study is to compare and evaluate the alveolar bone growth on maxillary sinus after bilateral sinus lift procedures, performed using PRF alone on one side and DFDBA with bio membrane on the other with the simultaneous placement of dental implants.</p> |
| − | <p style="text-indent:0pt; text-align:justify; margin-top:0.5em; margin-bottom:0.5em;"><span><b>Materials and Methods:</b></span> A total of twenty patients who needed implants in the maxillary posterior region with deficient maxillary ridge height were selected. All patients received only autologous PRF on one side and DFDBA material with bio-membrane on the contralateral side. All sinus augmentations were performed with the simultaneous placement of dental implants.</p> | + | <p style="text-indent:0pt; text-align:justify; margin-right: 2em; margin-left: 1.5em; margin-top:0.5em; margin-bottom:0.5em;"><span><b>Materials and Methods:</b></span> A total of twenty patients who needed implants in the maxillary posterior region with deficient maxillary ridge height were selected. All patients received only autologous PRF on one side and DFDBA material with bio-membrane on the contralateral side. All sinus augmentations were performed with the simultaneous placement of dental implants.</p> |
| − | <p style="text-indent:0pt; text-align:justify; margin-top:0.5em; margin-bottom:0.5em;"><span><b>Results:</b></span> Postoperative infection was not seen in any of the cases in both PRF and DFDBA (with bio-membrane) sides. Observation of slightly more bone formation and bone opacity with PRF than DFBDA with biomembrane was made. The thickness of the regenerated bone was similar to that of native bone on more sites on PRF group than that on the side of DFDBA. Comparing PRF alone and DFDBA with bio-membrane, a higher percentage of new bone formation on PRF sites and more implants were firm after 6 months on the side of PRF compared with that of DFDBA with bio-membrane side.</p> | + | <p style="text-indent:0pt; text-align:justify; margin-right: 2em; margin-left: 1.5em; margin-top:0.5em; margin-bottom:0.5em;"><span><b>Results:</b></span> Postoperative infection was not seen in any of the cases in both PRF and DFDBA (with bio-membrane) sides. Observation of slightly more bone formation and bone opacity with PRF than DFBDA with biomembrane was made. The thickness of the regenerated bone was similar to that of native bone on more sites on PRF group than that on the side of DFDBA. Comparing PRF alone and DFDBA with bio-membrane, a higher percentage of new bone formation on PRF sites and more implants were firm after 6 months on the side of PRF compared with that of DFDBA with bio-membrane side.</p> |
| − | <p style="text-indent:0pt; text-align:justify; margin-top:0.5em; margin-bottom:0.5em;"><span><b>Conclusion:</b></span> After assessing the results of both the materials, clinically and radiologically, it can be concluded that both PRF and DFDBA have given good results and showed greater ability in sinus lift procedures, only when they are compared with each other, PRF alone was superior to DFDBA.</p> | + | <p style="text-indent:0pt; text-align:justify; margin-right: 2em; margin-left: 1.5em; margin-top:0.5em; margin-bottom:0.5em;"><span><b>Conclusion:</b></span> After assessing the results of both the materials, clinically and radiologically, it can be concluded that both PRF and DFDBA have given good results and showed greater ability in sinus lift procedures, only when they are compared with each other, PRF alone was superior to DFDBA.</p> |
| − | <p style="text-indent:0pt; text-align:justify; margin-top:1.5em; margin-bottom:0.5em;"><span><b>Key words:</b></span> Sinus lift, implants, PRF, bone grafts.</p> | + | <p style="text-indent:1.5em; text-align:justify; margin-top:1.5em; margin-bottom:0.5em;"><span><b>Key Words:</b></span> Sinus lift, implants, PRF, bone grafts.</p> |
| | <hr class="part-rule"/> | | <hr class="part-rule"/> |
| | </font> | | </font> |
| | </div> | | </div> |
| − | <div class="body" id="article-body" onclick="void(0)";> | + | <div class="body" id="article-body"> |
| | <div> | | <div> |
| − | <font face="Tahoma" color="black" size="2"> | + | <font face="Arial" color="black" size="2"> |
| | <h3 class="title">Introduction</h3> | | <h3 class="title">Introduction</h3> |
| − | <p style="text-indent:1.5em; text-align:justify; margin-top:0.5em; margin-bottom:0.5em;">Anatomic limitations often associated with the posterior maxilla are a flat palatal vault, deficient alveolar height, inadequate posterior alveolus, increased pneumatization of the maxillary sinus and close approximation of the sinus to crestal bone. Following tooth loss, the edentulous alveolar process of the posterior usually undergo severe resorption. When teeth are extracted in the posterior maxilla, bone in that area is lost due to pneumatisation of the maxillary sinus.<a href="#ref1"><font face="Tahoma" size=".8">1</font></a> To overcome these problems, different solutions were proposed over the years, such as use of short implants, tilted implants placed in the anterior maxilla, zygoma implants and maxillary sinus floor elevation and grafting procedures with autogenous bone or allografts, xenografts and alloplastic materials. The traditional lateral approach using a Caldwell-luc osteotomy, the first main technique, where the maxillary sinus floor is grafted to provide a sufficient quantity of bone for the placement of endosteal dental implants.<a href="#ref1"><font face="Tahoma" size=".8">1</font></a> The objective of this approach is to use the natural osteogenic properties of the Schneiderian membrane to gain the missing millimetres of bone around the tips of the implants. Sinus elevation with autogenous bone grafts is considered to be the gold standard, many researchers have attempted to modify this procedure because of the morbidity associated with bone harvesting.</p> | + | <p style="text-indent:1.5em; text-align:justify; margin-top:0.5em; margin-bottom:0.5em;">Anatomic limitations often associated with the posterior maxilla are a flat palatal vault, deficient alveolar height, inadequate posterior alveolus, increased pneumatization of the maxillary sinus and close approximation of the sinus to crestal bone. Following tooth loss, the edentulous alveolar process of the posterior usually undergo severe resorption. When teeth are extracted in the posterior maxilla, bone in that area is lost due to pneumatisation of the maxillary sinus.<a href="#ref1"><font face="Arial" size=".8">1</font></a> To overcome these problems, different solutions were proposed over the years, such as use of short implants, tilted implants placed in the anterior maxilla, zygoma implants and maxillary sinus floor elevation and grafting procedures with autogenous bone or allografts, xenografts and alloplastic materials. The traditional lateral approach using a Caldwell-luc osteotomy, the first main technique, where the maxillary sinus floor is grafted to provide a sufficient quantity of bone for the placement of endosteal dental implants.<a href="#ref1"><font face="Arial" size=".8">1</font></a> The objective of this approach is to use the natural osteogenic properties of the Schneiderian membrane to gain the missing millimetres of bone around the tips of the implants. Sinus elevation with autogenous bone grafts is considered to be the gold standard, many researchers have attempted to modify this procedure because of the morbidity associated with bone harvesting.</p> |
| − | <p style="text-indent:1.5em; text-align:justify; margin-top:0.5em; margin-bottom:0.5em;">A new family of platelet concentrate, which is neither a fibrin glue nor a classical platelet concentrate, a new biomaterial, called platelet-rich fibrin (PRF), releases high amounts of growth factors TGF-b1, PDGF-AB etc.<a href="#ref2"><font face="Tahoma" size=".8">2</font></a>-<a href="#ref5"><font face="Tahoma" size=".8">5</font></a> No doubt that there is considerable osteogenic potential in schneiderian membrane and sinus periosteum, this when combined with PRF with presence of abundant growth factors can enhance adequate bone formation in the gap created by sinus lift procedure. The present study aimed to evaluate the potential of PRF alone to enhance bone regeneration after SFE in comparison with Demineralized freeze dried bone allograft (DFDBA) with bio membrane.</p> | + | <p style="text-indent:1.5em; text-align:justify; margin-top:0.5em; margin-bottom:0.5em;">A new family of platelet concentrate, which is neither a fibrin glue nor a classical platelet concentrate, a new biomaterial, called platelet-rich fibrin (PRF), releases high amounts of growth factors TGF-b1, PDGF-AB etc.<a href="#ref2"><font face="Arial" size=".8">2</font></a>-<a href="#ref5"><font face="Arial" size=".8">5</font></a> No doubt that there is considerable osteogenic potential in schneiderian membrane and sinus periosteum, this when combined with PRF with presence of abundant growth factors can enhance adequate bone formation in the gap created by sinus lift procedure. The present study aimed to evaluate the potential of PRF alone to enhance bone regeneration after SFE in comparison with Demineralized freeze dried bone allograft (DFDBA) with bio membrane.</p> |
| | </font> | | </font> |
| | </div> | | </div> |
| | <div> | | <div> |
| − | <font face="Tahoma" color="black" size="2"> | + | <font face="Arial" color="black" size="2"> |
| | <h3 class="title">Materials and Methods</h3> | | <h3 class="title">Materials and Methods</h3> |
| | <p style="text-indent:1.5em; text-align:justify; margin-top:0.5em; margin-bottom:0.5em;">A total of twenty patients who needed implants in the maxillary posterior region with deficient maxillary ridge height were selected. All patients received only autologous PRF on one side and demineralized freeze dried bone allograft (DFDBA) material with bio-membrane on the contralateral side. All sinus augmentations were performed with the simultaneous placement of dental implants. The selection criteria was as following: Patients with a residual alveolar ridge of less than 8 mm in the posterior atrophic maxilla, Cases in which sinus floor augmentation for at least one of the implant is indicated on both sides, without any systemic diseases and good general state of health, patients without any pathology affecting the maxillary sinus, correct inter-arch relationship.</p> | | <p style="text-indent:1.5em; text-align:justify; margin-top:0.5em; margin-bottom:0.5em;">A total of twenty patients who needed implants in the maxillary posterior region with deficient maxillary ridge height were selected. All patients received only autologous PRF on one side and demineralized freeze dried bone allograft (DFDBA) material with bio-membrane on the contralateral side. All sinus augmentations were performed with the simultaneous placement of dental implants. The selection criteria was as following: Patients with a residual alveolar ridge of less than 8 mm in the posterior atrophic maxilla, Cases in which sinus floor augmentation for at least one of the implant is indicated on both sides, without any systemic diseases and good general state of health, patients without any pathology affecting the maxillary sinus, correct inter-arch relationship.</p> |
| | <p style="text-indent:1.5em; text-align:justify; margin-top:0.5em; margin-bottom:0.5em;">Patients excluded from the study were patients with uncontrolled systemic diseases, ongoing chemotherapy, radiation therapy or maxillary sinus disease, immuno-compromised patients. Patients with untreated periodontitis, lack of opposing dentition/prosthesis, acute or chronic infection or inflammation in the area intended for implant placement, and chronic smokers who are not able to quit the habit.</p> | | <p style="text-indent:1.5em; text-align:justify; margin-top:0.5em; margin-bottom:0.5em;">Patients excluded from the study were patients with uncontrolled systemic diseases, ongoing chemotherapy, radiation therapy or maxillary sinus disease, immuno-compromised patients. Patients with untreated periodontitis, lack of opposing dentition/prosthesis, acute or chronic infection or inflammation in the area intended for implant placement, and chronic smokers who are not able to quit the habit.</p> |
| | <p style="text-indent:1.5em; text-align:justify; margin-top:0.5em; margin-bottom:0.5em;">The assessment was done pre-operatively by obtaining an OPG, and a CT scan of 0.6 mm sections (DENTASCAN). The bone height was measured using the CT Scans along with the bone density in terms of Hounsfield units and an implant was chosen accordingly. At 6 months post-operatively the assessment of the bone height was done using a CT scan. The bone density was also measured on the CT scans in terms of Hounsfield units. All sinus lift procedures were performed using direct sinus lift method (lateral antrostomy). A preoperative panoramic radiograph was taken to evaluate the bone height before sinus lift. The patients were treated under local anesthesia and proper asepsis was maintained. The buccal window technique was used. The bone was removed and the schneiderian membrane was exposed and elevated from bone. The integrity of the sinus membrane was checked by its movement while the patient was breathing. Adequate space was created for implants. For small perforations in the membrane, up to the size of 2mm a resorbable collagen membrane was placed in the area to adapt to the perforation, occlude it and allow it time to heal and repair itself.</p> | | <p style="text-indent:1.5em; text-align:justify; margin-top:0.5em; margin-bottom:0.5em;">The assessment was done pre-operatively by obtaining an OPG, and a CT scan of 0.6 mm sections (DENTASCAN). The bone height was measured using the CT Scans along with the bone density in terms of Hounsfield units and an implant was chosen accordingly. At 6 months post-operatively the assessment of the bone height was done using a CT scan. The bone density was also measured on the CT scans in terms of Hounsfield units. All sinus lift procedures were performed using direct sinus lift method (lateral antrostomy). A preoperative panoramic radiograph was taken to evaluate the bone height before sinus lift. The patients were treated under local anesthesia and proper asepsis was maintained. The buccal window technique was used. The bone was removed and the schneiderian membrane was exposed and elevated from bone. The integrity of the sinus membrane was checked by its movement while the patient was breathing. Adequate space was created for implants. For small perforations in the membrane, up to the size of 2mm a resorbable collagen membrane was placed in the area to adapt to the perforation, occlude it and allow it time to heal and repair itself.</p> |
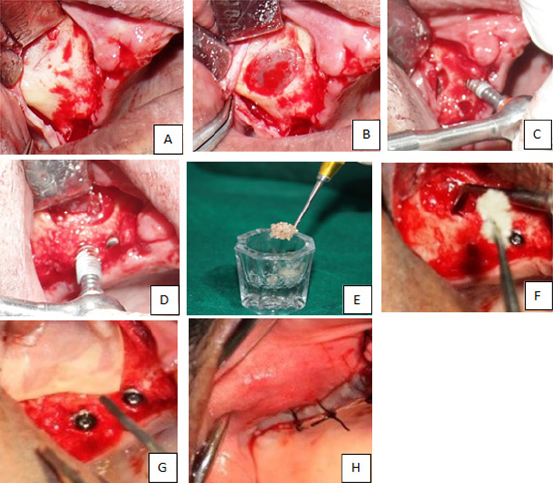
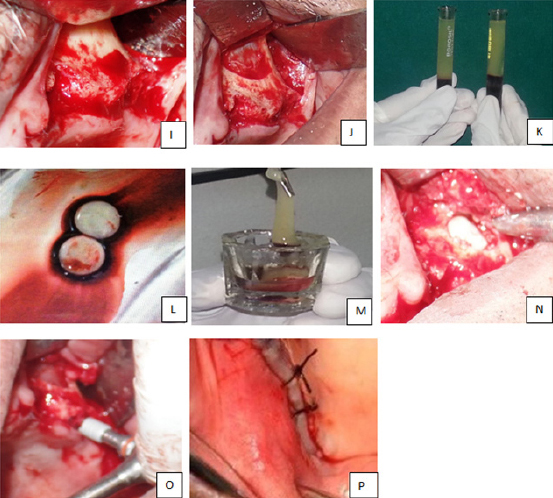
| − | <p style="text-indent:1.5em; text-align:justify; margin-top:0.5em; margin-bottom:0.5em;">Once the desired amount of sinus membrane elevation was achieved, the defects were then filled with DFDBA on one side and PRF on the other. A particular precaution was taken while filling the defect with DFDBA. The graft was first placed in the normal saline for some time and then the medial aspect of the sinus cavity was filled prior to the placement of implant. Implant was then placed and the rest of the space was loosely filled with remaining graft material. The periocol membrane was gently placed over the lateral window and gently but firmly tucked under the periosteum to help in preventing the graft material escaping through the defect and also prevent in-growth of connective tissue into the sinus cavity (<a href="#F1"><font face="Tahoma" size=".8">Figure 1</font></a>). On the PRF side, following the preparation of implant site, the sinus cavity was first filled with PRF (<a href="#F2"><font face="Tahoma" size=".8">Figure 2</font></a>) and then the implants were placed simultaneously in the same appointment, and the flap was closed using resorbable 3-0 vicryl suture material. Patients were also advised not to blow their noses, engage in strenuous physical activities and, prescribed nasal decongestants for first two weeks. Post-operatively patients were reviewed at 1 week, and 6 months intervals. Parameters checked by radiographs and CT were, density of newly formed bone in a grafted area, consolidation, assessment of interface between the implant and the newly formed bone, the extent of the sinus floor elevation on radiographs, infection, stability of an implant.</p> | + | <p style="text-indent:1.5em; text-align:justify; margin-top:0.5em; margin-bottom:0.5em;">Once the desired amount of sinus membrane elevation was achieved, the defects were then filled with DFDBA on one side and PRF on the other. A particular precaution was taken while filling the defect with DFDBA. The graft was first placed in the normal saline for some time and then the medial aspect of the sinus cavity was filled prior to the placement of implant. Implant was then placed and the rest of the space was loosely filled with remaining graft material. The periocol membrane was gently placed over the lateral window and gently but firmly tucked under the periosteum to help in preventing the graft material escaping through the defect and also prevent in-growth of connective tissue into the sinus cavity (<a class="ref" href="http://nacd.in/ijda/08/04/images/IJDA-8-207-g001.jpg" target="_blank" >Figure 1</a>). On the PRF side, following the preparation of implant site, the sinus cavity was first filled with PRF (<a class="ref" href="http://nacd.in/ijda/08/04/images/IJDA-8-207-g002.jpg" target="_blank" >Figure 2</a>) and then the implants were placed simultaneously in the same appointment, and the flap was closed using resorbable 3-0 vicryl suture material. Patients were also advised not to blow their noses, engage in strenuous physical activities and, prescribed nasal decongestants for first two weeks. Post-operatively patients were reviewed at 1 week, and 6 months intervals. Parameters checked by radiographs and CT were, density of newly formed bone in a grafted area, consolidation, assessment of interface between the implant and the newly formed bone, the extent of the sinus floor elevation on radiographs, infection, stability of an implant.</p> |
| − | <div class="ienlarger"><a id="F1"><img class="resize_thumb" alt="thumb" src="http://nacd.in/images/0804/IJDA-8-207-g001.jpg"><span><img alt="large" src="http://nacd.in/images/0804/IJDA-8-207-g001.jpg"></img></span></img></a></div> | + | <table width="100%" cellpadding="1" cellspacing="1"> |
| − | <p style="text-indent:0pt; text-align:justify; margin-top:0.5em; margin-bottom:1.5em;"><span><b>Figure 1:</b></span> Direct sinus lift : Intraoperative procedure of right side: DFDBA with bio-membrane, (A) incision and reflection of mucoperiosteal flap; (B) Exposed sinus membrane; (C, D) placement of implants; € Bone graft; (F) Placement of bone graft; (G) Placing membrane; (H) Flap sutured.</p> | + | <tr> |
| − | <div class="ienlarger"><a id="F2"><img class="resize_thumb" alt="thumb" src="http://nacd.in/images/0804/IJDA-8-207-g002.jpg"><span><img alt="large" src="http://nacd.in/images/0804/IJDA-8-207-g002.jpg"></img></span></img></a></div> | + | <td align='center' bgcolor="f3f3f3" width='200px'><img src='http://nacd.in/ijda/08/04/images/IJDA-8-207-g001.jpg' height="95" alt='' style="padding:3px;"/> |
| − | <p style="text-indent:0pt; text-align:justify; margin-top:0.5em; margin-bottom:1.5em;"><span><b>Figure 2:</b></span> Intra-operative procedure on left side PRF alone: (I, J) Incision given, mucoperiosteal flap reflected and preparation of the antrostomy site done; (K, L, M) Sinus elevation and PRF preparation done and taken into a sterile container; (N) PRF inserted into the space created after the elevation of the sinus membrane; (O) Implants placement is done in the molar region; (P) Closure</p> | + | <td bgcolor="eaeaea" style="padding:5px;"> |
| | + | <font class='ref'>Figure 1: Direct sinus lift : Intraoperative procedure of right side: DFDBA with bio-membrane, (A) incision and reflection of mucoperiosteal flap; (B) Exposed sinus membrane; (C, D) placement of implants; € Bone graft; (F) Placement of bone graft; (G) Placing membrane; (H) Flap sutured.</font> |
| | + | <br> |
| | + | <br> |
| | + | <a class="ref" href="http://nacd.in/ijda/08/04/images/IJDA-8-207-g001.jpg" target="_blank"> |
| | + | <b>Click here to view</b> |
| | + | </a> |
| | + | </td> |
| | + | </tr> |
| | + | </table> |
| | + | <table width="100%" cellpadding="1" cellspacing="1"> |
| | + | <tr> |
| | + | <td align='center' bgcolor="f3f3f3" width='200px'><img src='http://nacd.in/ijda/08/04/images/IJDA-8-207-g002.jpg' height="95" alt='' style="padding:3px;"/> |
| | + | <td bgcolor="eaeaea" style="padding:5px;"> |
| | + | <font class='ref'>Figure 2: Intra-operative procedure on left side PRF alone: (I, J) Incision given, mucoperiosteal flap reflected and preparation of the antrostomy site done; (K, L, M) Sinus elevation and PRF preparation done and taken into a sterile container; (N) PRF inserted into the space created after the elevation of the sinus membrane; (O) Implants placement is done in the molar region; (P) Closure</font> |
| | + | <br> |
| | + | <br> |
| | + | <a class="ref" href="http://nacd.in/ijda/08/04/images/IJDA-8-207-g002.jpg" target="_blank"> |
| | + | <b>Click here to view</b> |
| | + | </a> |
| | + | </td> |
| | + | </tr> |
| | + | </table> |
| | </font> | | </font> |
| | </div> | | </div> |
| | <div> | | <div> |
| − | <font face="Tahoma" color="black" size="2"> | + | <font face="Arial" color="black" size="2"> |
| | <h3 class="title">Observations and Results</h3> | | <h3 class="title">Observations and Results</h3> |
| | <p style="text-indent:1.5em; text-align:justify; margin-top:0.5em; margin-bottom:0.5em;">Out of 20 patients included in the study 10 were male and 10 were female. All the patients were treated with PRF alone on one side and DFDBA with bio-membrane on the other side. Evaluation and Indexing of results:</p> | | <p style="text-indent:1.5em; text-align:justify; margin-top:0.5em; margin-bottom:0.5em;">Out of 20 patients included in the study 10 were male and 10 were female. All the patients were treated with PRF alone on one side and DFDBA with bio-membrane on the other side. Evaluation and Indexing of results:</p> |
| Line 101: |
Line 121: |
| | </div> | | </div> |
| | <div> | | <div> |
| − | <font face="Tahoma" color="black" size="2"> | + | <font face="Arial" color="black" size="2"> |
| | <h3 class="title">Discussion</h3> | | <h3 class="title">Discussion</h3> |
| − | <p style="text-indent:1.5em; text-align:justify; margin-top:0.5em; margin-bottom:0.5em;">The rehabilitation of posterior edentolous maxilla with implants means the surgeon has to face with inadequate bone in the area of interest. Such situations warrant for a direct sinus lift procedure. Many algorithms have been suggested in such situations, but the one that drew best attention was given by Testori et.al in 2012. The next question that arise is what type of graft material to be used in the area created by DSL (Direct Sinus Lift) procedure. The best materials which have been used from times immemorial include autogenous bone, bone allografts, xenografts, and alloplast such as tricalcium phosphate (TCP), resorbable and nonresorbable hydroxyapatite (HA), bovine-derived bone mineral, and bioactive glasses. The first paper concerning the treatment of patients with endosseous implants associated with maxillary sinus elevation operations was published in 1980 by Boyne and James.<a href="#ref6"><font face="Tahoma" size=".8">6</font></a> Over the years various techniques have been proposed for maxillary sinus augmentation which differ in the surgical protocol (the method and anatomic site for carrying out the antrostomy), in the autogenous bone harvesting site, in the type of graft material used, in the timing of the implant surgery in relation to maxillary sinus floor elevation (simultaneous i.e. during the same surgical procedure or later), use or non-use of resorbable or non resorbable membranes and in the extent of the elevation of the sinus membrane . The use of cortical and cortico-cancellous blocks adapted to the sinus floor also has been reported, although particulated graft material may reduce healing time. DFDBA has had a long and successful history of use as a grafting material in periodontal defects. The utilization of DFDBA as a sinus grafting material, however, has not demonstrated this level of success. Results from the Sinus Consensus Conference show poor results as evidenced by lower implant survival rates when used alone (85%) or in combination with autografts and xenografts (82% and 80%, respectively).<a href="#ref7"><font face="Tahoma" size=".8">7</font></a> A histological report and a clinical report demonstrate both poor-quality bone formation and a relatively low implant survival rate (80%).<a href="#ref8"><font face="Tahoma" size=".8">8</font></a> Turnover of this graft material to vital bone proceeds slowly, as a non-vital remineralization precedes vital bone formation. Urist suggested that allografts form bone by osteoinduction because they contain osteoinductive proteins called bone morphogenetic proteins (BMP).<a href="#ref9"><font face="Tahoma" size=".8">9</font></a> The most commonly used forms of allografts are frozen freeze dried, and irradiated. Fresh allografts are the most antigenic; freezing or freeze drying the bone significantly reduces the antigenicity. Clinical experience has shown that grafting of the sinuses with DFDBA alone results in the presence of dense connective tissue after 6 months, whereas grafting with FDBA results in the presence of new bone formation. Compared to other bone regeneration materials DFDBA has the advantage of rapid resorption and exposure of osteoinductive proteins following demineralization.<a href="#ref10"><font face="Tahoma" size=".8">10</font></a></p> | + | <p style="text-indent:1.5em; text-align:justify; margin-top:0.5em; margin-bottom:0.5em;">The rehabilitation of posterior edentolous maxilla with implants means the surgeon has to face with inadequate bone in the area of interest. Such situations warrant for a direct sinus lift procedure. Many algorithms have been suggested in such situations, but the one that drew best attention was given by Testori et.al in 2012. The next question that arise is what type of graft material to be used in the area created by DSL (Direct Sinus Lift) procedure. The best materials which have been used from times immemorial include autogenous bone, bone allografts, xenografts, and alloplast such as tricalcium phosphate (TCP), resorbable and nonresorbable hydroxyapatite (HA), bovine-derived bone mineral, and bioactive glasses. The first paper concerning the treatment of patients with endosseous implants associated with maxillary sinus elevation operations was published in 1980 by Boyne and James.<a href="#ref6"><font face="Arial" size=".8">6</font></a> Over the years various techniques have been proposed for maxillary sinus augmentation which differ in the surgical protocol (the method and anatomic site for carrying out the antrostomy), in the autogenous bone harvesting site, in the type of graft material used, in the timing of the implant surgery in relation to maxillary sinus floor elevation (simultaneous i.e. during the same surgical procedure or later), use or non-use of resorbable or non resorbable membranes and in the extent of the elevation of the sinus membrane . The use of cortical and cortico-cancellous blocks adapted to the sinus floor also has been reported, although particulated graft material may reduce healing time. DFDBA has had a long and successful history of use as a grafting material in periodontal defects. The utilization of DFDBA as a sinus grafting material, however, has not demonstrated this level of success. Results from the Sinus Consensus Conference show poor results as evidenced by lower implant survival rates when used alone (85%) or in combination with autografts and xenografts (82% and 80%, respectively).<a href="#ref7"><font face="Arial" size=".8">7</font></a> A histological report and a clinical report demonstrate both poor-quality bone formation and a relatively low implant survival rate (80%).<a href="#ref8"><font face="Arial" size=".8">8</font></a> Turnover of this graft material to vital bone proceeds slowly, as a non-vital remineralization precedes vital bone formation. Urist suggested that allografts form bone by osteoinduction because they contain osteoinductive proteins called bone morphogenetic proteins (BMP).<a href="#ref9"><font face="Arial" size=".8">9</font></a> The most commonly used forms of allografts are frozen freeze dried, and irradiated. Fresh allografts are the most antigenic; freezing or freeze drying the bone significantly reduces the antigenicity. Clinical experience has shown that grafting of the sinuses with DFDBA alone results in the presence of dense connective tissue after 6 months, whereas grafting with FDBA results in the presence of new bone formation. Compared to other bone regeneration materials DFDBA has the advantage of rapid resorption and exposure of osteoinductive proteins following demineralization.<a href="#ref10"><font face="Arial" size=".8">10</font></a></p> |
| − | <p style="text-indent:1.5em; text-align:justify; margin-top:0.5em; margin-bottom:0.5em;">In the present study, demineralized freeze dried bone allograft, size of granules more than 1040 μ, sterilized by gamma radiation was used. The effects of PRF in treating maxillary sinus are remarkable. Choukroun et al in their research on maxillary sinus lift involving PRF and a lateral approach, used freeze-dried bone allograft (FDBA) and PRF.<a href="#ref5"><font face="Tahoma" size=".8">5</font></a> When performing a maxillary sinus lift using PRF, there has been a report that indicates the healing period can be reduced by about 4 months. Also, in the same manner as observed in alveolar bone graft surgery, PRF can protect the maxillary sinus lateral window. To promote the healing recently, a method was suggested where only PRF would be used in a maxillary sinus lift without any graft material. Mazor et al used a lateral approach to lift the lower part of maxillary sinus, simultaneously placed an implant, and only placed PRF.<a href="#ref4"><font face="Tahoma" size=".8">4</font></a> Acquired bone height was 10.1±0.9 mm on average, and they reported that there were no implant failures. Inside the maxillary sinus, PRF not only prevents damage to the maxillary sinus membrane, it also promotes bone formation and can help maintain the height of the lifted maxillary sinus for a certain period of time. In experiments conducted by Choi et al the maxillary sinus membrane was intentionally perforated about 2 mm, received fibrin glue and collagen membrane, and was histologically analysed.<a href="#ref11"><font face="Tahoma" size=".8">11</font></a> The maxillary sinus membrane was restored to a continuous structure as before in fibrin glue group, but inflammation and infiltration of fibrotic tissues were observed in collagen membrane group. When we examine the study in which the maxillary sinus membrane is perforated more than 2 mm, the results indicate that for bone of lateral gums, the lateral window can be covered by a PRF membrane.<a href="#ref12"><font face="Tahoma" size=".8">12</font></a></p> | + | <p style="text-indent:1.5em; text-align:justify; margin-top:0.5em; margin-bottom:0.5em;">In the present study, demineralized freeze dried bone allograft, size of granules more than 1040 μ, sterilized by gamma radiation was used. The effects of PRF in treating maxillary sinus are remarkable. Choukroun et al in their research on maxillary sinus lift involving PRF and a lateral approach, used freeze-dried bone allograft (FDBA) and PRF.<a href="#ref5"><font face="Arial" size=".8">5</font></a> When performing a maxillary sinus lift using PRF, there has been a report that indicates the healing period can be reduced by about 4 months. Also, in the same manner as observed in alveolar bone graft surgery, PRF can protect the maxillary sinus lateral window. To promote the healing recently, a method was suggested where only PRF would be used in a maxillary sinus lift without any graft material. Mazor et al used a lateral approach to lift the lower part of maxillary sinus, simultaneously placed an implant, and only placed PRF.<a href="#ref4"><font face="Arial" size=".8">4</font></a> Acquired bone height was 10.1±0.9 mm on average, and they reported that there were no implant failures. Inside the maxillary sinus, PRF not only prevents damage to the maxillary sinus membrane, it also promotes bone formation and can help maintain the height of the lifted maxillary sinus for a certain period of time. In experiments conducted by Choi et al the maxillary sinus membrane was intentionally perforated about 2 mm, received fibrin glue and collagen membrane, and was histologically analysed.<a href="#ref11"><font face="Arial" size=".8">11</font></a> The maxillary sinus membrane was restored to a continuous structure as before in fibrin glue group, but inflammation and infiltration of fibrotic tissues were observed in collagen membrane group. When we examine the study in which the maxillary sinus membrane is perforated more than 2 mm, the results indicate that for bone of lateral gums, the lateral window can be covered by a PRF membrane.<a href="#ref12"><font face="Arial" size=".8">12</font></a></p> |
| | <p style="text-indent:1.5em; text-align:justify; margin-top:0.5em; margin-bottom:0.5em;">The present study aimed at evaluating the efficacy of PRF alone to enhance bone regeneration after SFE in comparison with DFDBA with bio-membrane. The regenerative ability of the DFDBA was used as a tool to assess the prognostic value of PRF.</p> | | <p style="text-indent:1.5em; text-align:justify; margin-top:0.5em; margin-bottom:0.5em;">The present study aimed at evaluating the efficacy of PRF alone to enhance bone regeneration after SFE in comparison with DFDBA with bio-membrane. The regenerative ability of the DFDBA was used as a tool to assess the prognostic value of PRF.</p> |
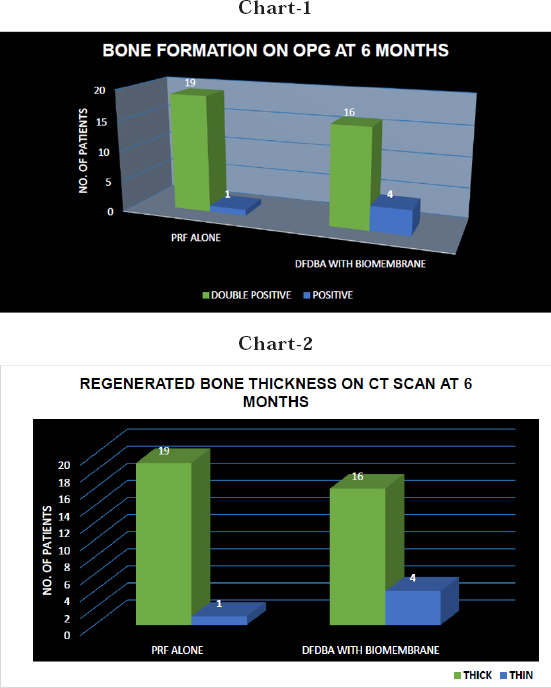
| − | <p style="text-indent:1.5em; text-align:justify; margin-top:0.5em; margin-bottom:0.5em;">In the present study the alveolar bone growth was compared in the two sides of the maxillary sinus after bilateral sinus lift procedures performed with the simultaneous placement of dental implants in 20 consecutive patients. The radiographical assessment was done by an orthopantomogram and a CT scan (Dentascan of 0.6 mm sections). All the patients included in the study were with a bone height of less than 8 mm who were treated using a direct sinus lift procedure. The mean bone height of the all patients (pre-operatively) who were treated with a direct sinus lift was 6.0 mm on the right side and 5.8 on the left side and the heights varied anywhere between 3.5 mm to 7.4 mm for a total of 50 implant sites. All implant sites were clinically evaluated for their stability by, the implants were exposed by a tissue puncture. Then the implant stability was checked by a sterile probe by moving it mesio-distally and bucco-lingually. Any movement of the implant was considered as non- osseointegration. A zero movement indicated a firm and stable implant. Clinically, among 20 sites of PRF, implants on 19 sites were clinically firm while implant on 1 side was mobile whereas among 20 sites of DFDBA, implants on 16 sites were firm and stable whereas implants on 4 sites were mobile (Table: XI) Each implant was assessed 6 months post-op by an IOPA. The IOPA was chosen because it gives more or less accurate details of the implant and bone interface (<a href="#F3"><font face="Tahoma" size=".8">Figure 3</font></a>). Any interface less than 1 mm is considered as an osseointegrated and successful implant. Only 1 implant on the side of PRF were mobile with a bone -implant interface of >1mm whereas on the other side i.e. On the side of DFDBA 4 implants were mobile with a bone-implant interface showing >1mm. In addition to this post-op CT scan taken 6 months later has given us a perfect picture on the bone-implant interface. The CT scan offered us the best marginal definition of the osseointegrated implants. We have evaluated the amount of bone gain both on the OPG’s and CT Scans (<a href="#F4"><font face="Tahoma" size=".8">Figure 4</font></a>). While the results in the OPG are not predictable more emphasis was given on the CT scans. On OPG the magnification error was removed as we already know the length of the implant. The amount of bone opacity apical to the implant from the most apical point of the implant to the most apical point of the bone opacity was taken into consideration after deducting the magnification error. This gave the bone gain in an OPG. The bone gain was accurately measured on CT Scans. The method was similar to what we followed on an OPG, except that we didn’t have to calculate the magnification error as it is irrelevant on CT Scans. The mean bone gain was 5.91mm on the side of PRF and 5.01mm on the side of DFDBA. There were 3 implant sites with a bone height between 3.0 - 3.9 mm of which implants of size 5.3 x 10 mm (2) and an implant size of 3.5X 8mm were used. There were 11 implant sites with a bone height between 4.0 - 4.9 mm and implants of size 3.7 x 10 mm (3), 4.3 x 10 mm (2), 5.3 x 10 mm (4), 4.2 x11.5 (2) were used. There were 14 implant sites with a bone height between 5.0 - 5.9 mm of which implants of size 5.3 x 10 (4), 3. 7 x 10 (3), 3.7 x 13 (3), 4.3 x 10 (4), were used placing a total of 14 implants in such sites. There were 16 implant sites with a bone height between 6.0 - 6.9 mm and implants of size 3.7 x 13(4), 4.3 x 13(6), 5.3 x 10(4), 4.3 x 10(2) and a total of 16 implants were placed in these sites. There were 6 implant sites with a bone height between 7.0 - 7.9 mm and implants of the sizes 4.3 x 10 (2) 5.3 x 10 (2) and 4.3 x 13 (2) were used placing a total of 6 implants. Radiographically on an OPG the bone opacity was close or sometimes more than that of native bone on 19 sites of PRF and was less than that of native bone on 1 site postoperatively after 6 months. Whereas the bone opacity was closer on 16 sites and less than that of native bone on 4 sites of DFDBA. Panoramic results showed enhancement of bone regeneration on 19 sites of PRF and 16 sites of DFDBA at 6<sup>th</sup> month postoperatively. The CT findings (<a href="#F4"><font face="Tahoma" size=".8">Figure 4</font></a>) were more specific. The examination and evaluation involved both morphological analysis and bone density analysis in Hounsfield units. All cases were subjected to CT scan at 6<sup>th</sup> month post operatively.<a href="#ref13"><font face="Tahoma" size=".8">13</font></a> In the present study, cancellous bone structures on panoramic, axial and buccolingual view images were observed.</p> | + | <p style="text-indent:1.5em; text-align:justify; margin-top:0.5em; margin-bottom:0.5em;">In the present study the alveolar bone growth was compared in the two sides of the maxillary sinus after bilateral sinus lift procedures performed with the simultaneous placement of dental implants in 20 consecutive patients. The radiographical assessment was done by an orthopantomogram and a CT scan (Dentascan of 0.6 mm sections). All the patients included in the study were with a bone height of less than 8 mm who were treated using a direct sinus lift procedure. The mean bone height of the all patients (pre-operatively) who were treated with a direct sinus lift was 6.0 mm on the right side and 5.8 on the left side and the heights varied anywhere between 3.5 mm to 7.4 mm for a total of 50 implant sites. All implant sites were clinically evaluated for their stability by, the implants were exposed by a tissue puncture. Then the implant stability was checked by a sterile probe by moving it mesio-distally and bucco-lingually. Any movement of the implant was considered as non- osseointegration. A zero movement indicated a firm and stable implant. Clinically, among 20 sites of PRF, implants on 19 sites were clinically firm while implant on 1 side was mobile whereas among 20 sites of DFDBA, implants on 16 sites were firm and stable whereas implants on 4 sites were mobile (Table: XI) Each implant was assessed 6 months post-op by an IOPA. The IOPA was chosen because it gives more or less accurate details of the implant and bone interface (<a class="ref" href="http://nacd.in/ijda/08/04/images/IJDA-8-207-g003.jpg" target="_blank" >Figure 3</a>). Any interface less than 1 mm is considered as an osseointegrated and successful implant. Only 1 implant on the side of PRF were mobile with a bone -implant interface of >1mm whereas on the other side i.e. On the side of DFDBA 4 implants were mobile with a bone-implant interface showing >1mm. In addition to this post-op CT scan taken 6 months later has given us a perfect picture on the bone-implant interface. The CT scan offered us the best marginal definition of the osseointegrated implants. We have evaluated the amount of bone gain both on the OPG’s and CT Scans (<a class="ref" href="http://nacd.in/ijda/08/04/images/IJDA-8-207-g004.jpg" target="_blank" >Figure 4</a>). While the results in the OPG are not predictable more emphasis was given on the CT scans. On OPG the magnification error was removed as we already know the length of the implant. The amount of bone opacity apical to the implant from the most apical point of the implant to the most apical point of the bone opacity was taken into consideration after deducting the magnification error. This gave the bone gain in an OPG. The bone gain was accurately measured on CT Scans. The method was similar to what we followed on an OPG, except that we didn’t have to calculate the magnification error as it is irrelevant on CT Scans. The mean bone gain was 5.91mm on the side of PRF and 5.01mm on the side of DFDBA. There were 3 implant sites with a bone height between 3.0 - 3.9 mm of which implants of size 5.3 x 10 mm (2) and an implant size of 3.5X 8mm were used. There were 11 implant sites with a bone height between 4.0 - 4.9 mm and implants of size 3.7 x 10 mm (3), 4.3 x 10 mm (2), 5.3 x 10 mm (4), 4.2 x11.5 (2) were used. There were 14 implant sites with a bone height between 5.0 - 5.9 mm of which implants of size 5.3 x 10 (4), 3. 7 x 10 (3), 3.7 x 13 (3), 4.3 x 10 (4), were used placing a total of 14 implants in such sites. There were 16 implant sites with a bone height between 6.0 - 6.9 mm and implants of size 3.7 x 13(4), 4.3 x 13(6), 5.3 x 10(4), 4.3 x 10(2) and a total of 16 implants were placed in these sites. There were 6 implant sites with a bone height between 7.0 - 7.9 mm and implants of the sizes 4.3 x 10 (2) 5.3 x 10 (2) and 4.3 x 13 (2) were used placing a total of 6 implants. Radiographically on an OPG the bone opacity was close or sometimes more than that of native bone on 19 sites of PRF and was less than that of native bone on 1 site postoperatively after 6 months. Whereas the bone opacity was closer on 16 sites and less than that of native bone on 4 sites of DFDBA. Panoramic results showed enhancement of bone regeneration on 19 sites of PRF and 16 sites of DFDBA at 6<sup>th</sup> month postoperatively. The CT findings (<a class="ref" href="http://nacd.in/ijda/08/04/images/IJDA-8-207-g004.jpg" target="_blank" >Figure 4</a>) were more specific. The examination and evaluation involved both morphological analysis and bone density analysis in Hounsfield units. All cases were subjected to CT scan at 6<sup>th</sup> month post operatively.<a href="#ref13"><font face="Arial" size=".8">13</font></a> In the present study, cancellous bone structures on panoramic, axial and buccolingual view images were observed.</p> |
| − | <div class="ienlarger"><a id="F3"><img class="resize_thumb" alt="thumb" src="http://nacd.in/images/0804/IJDA-8-207-g003.jpg"><span><img alt="large" src="http://nacd.in/images/0804/IJDA-8-207-g003.jpg"></img></span></img></a></div> | + | <table width="100%" cellpadding="1" cellspacing="1"> |
| − | <p style="text-indent:0pt; text-align:justify; margin-top:0.5em; margin-bottom:1.5em;"><span><b>Figure 3:</b></span> OPG and IOPAs showing bone formation in relation to implants after 6 months in both the groups.</p> | + | <tr> |
| − | <div class="ienlarger"><a id="F4"><img class="resize_thumb" alt="thumb" src="http://nacd.in/images/0804/IJDA-8-207-g001.jpg"><span><img alt="large" src="http://nacd.in/images/0804/IJDA-8-207-g001.jpg"></img></span></img></a></div> | + | <td align='center' bgcolor="f3f3f3" width='200px'><img src='http://nacd.in/ijda/08/04/images/IJDA-8-207-g003.jpg' height="95" alt='' style="padding:3px;"/> |
| − | <p style="text-indent:0pt; text-align:justify; margin-top:0.5em; margin-bottom:1.5em;"><span><b>Figure 4:</b></span> Charts 1 & 2 showing bone formation on OPG and bone thickness on CT scan in two groups.</p> | + | <td bgcolor="eaeaea" style="padding:5px;"> |
| | + | <font class='ref'>Figure 3: OPG and IOPAs showing bone formation in relation to implants after 6 months in both the groups.</font> |
| | + | <br> |
| | + | <br> |
| | + | <a class="ref" href="http://nacd.in/ijda/08/04/images/IJDA-8-207-g003.jpg" target="_blank"> |
| | + | <b>Click here to view</b> |
| | + | </a> |
| | + | </td> |
| | + | </tr> |
| | + | </table> |
| | + | <table width="100%" cellpadding="1" cellspacing="1"> |
| | + | <tr> |
| | + | <td align='center' bgcolor="f3f3f3" width='200px'><img src='http://nacd.in/ijda/08/04/images/IJDA-8-207-g004.jpg' height="95" alt='' style="padding:3px;"/> |
| | + | <td bgcolor="eaeaea" style="padding:5px;"> |
| | + | <font class='ref'>Figure 4: Charts 1 & 2 showing bone formation on OPG and bone thickness on CT scan in two groups.</font> |
| | + | <br> |
| | + | <br> |
| | + | <a class="ref" href="http://nacd.in/ijda/08/04/images/IJDA-8-207-g004.jpg" target="_blank"> |
| | + | <b>Click here to view</b> |
| | + | </a> |
| | + | </td> |
| | + | </tr> |
| | + | </table> |
| | </font> | | </font> |
| | </div> | | </div> |
| | <div> | | <div> |
| − | <font face="Tahoma" color="black" size="2"> | + | <font face="Arial" color="black" size="2"> |
| | <h3 class="title">CT Value Measurement</h3> | | <h3 class="title">CT Value Measurement</h3> |
| | <p style="text-indent:1.5em; text-align:justify; margin-top:0.5em; margin-bottom:0.5em;">The findings are significant. Out of 20 cases, the regenerated bone was similar to the native bone and thick in 19 cases on the side of PRF. On the side of DFDBA the regenerated bone was thick in 16 cases, thin as a whole in 4 cases.</p> | | <p style="text-indent:1.5em; text-align:justify; margin-top:0.5em; margin-bottom:0.5em;">The findings are significant. Out of 20 cases, the regenerated bone was similar to the native bone and thick in 19 cases on the side of PRF. On the side of DFDBA the regenerated bone was thick in 16 cases, thin as a whole in 4 cases.</p> |
| Line 122: |
Line 164: |
| | </div> | | </div> |
| | <div> | | <div> |
| − | <font face="Tahoma" color="black" size="2"> | + | <font face="Arial" color="black" size="2"> |
| | <h3 class="title">Conclusion</h3> | | <h3 class="title">Conclusion</h3> |
| | <p style="text-indent:1.5em; text-align:justify; margin-top:0.5em; margin-bottom:0.5em;">In the present study, after assessing the results of both the materials, clinically and radiologically, it can be concluded that both PRF and DFDBA have given good results and showed greater ability in sinus lift procedures, only when they are compared with each other, PRF alone was superior to DFDBA. The study also showed that PRF enhances the physiologic healing phenomenon. However, further research and large studies are required to look into platelet and inflammatory features of this biomaterial.</p> | | <p style="text-indent:1.5em; text-align:justify; margin-top:0.5em; margin-bottom:0.5em;">In the present study, after assessing the results of both the materials, clinically and radiologically, it can be concluded that both PRF and DFDBA have given good results and showed greater ability in sinus lift procedures, only when they are compared with each other, PRF alone was superior to DFDBA. The study also showed that PRF enhances the physiologic healing phenomenon. However, further research and large studies are required to look into platelet and inflammatory features of this biomaterial.</p> |
| Line 133: |
Line 175: |
| | <h3 class="title">References</h3> | | <h3 class="title">References</h3> |
| | <div> | | <div> |
| − | <p class="ref-label"><font face="Tahoma" color="black" size="1"><a name="ref1">1.</a> Summers R.B.; A new concept in maxillary implant surgery: the osteotome technique. Compendium 1994; 15(2)154-6.</font></p> | + | <p class="ref-label"><font face="Arial" color="black" size="1"><a name="ref1">1.</a> Summers R.B.; A new concept in maxillary implant surgery: the osteotome technique. Compendium 1994; 15(2)154-6.</font></p> |
| − | <p class="ref-label"><font face="Tahoma" color="black" size="1"><a name="ref2">2.</a> David M Dohan, Joseph Choukroun, Antoine Diss et al. Platelet-rich fibrin (PRF): A second-generation platelet concentrate.Part I: Technological concepts and evolution. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2006; 101(3):37-44.</font></p> | + | <p class="ref-label"><font face="Arial" color="black" size="1"><a name="ref2">2.</a> David M Dohan, Joseph Choukroun, Antoine Diss et al. Platelet-rich fibrin (PRF): A second-generation platelet concentrate.Part I: Technological concepts and evolution. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2006; 101(3):37-44.</font></p> |
| − | <p class="ref-label"><font face="Tahoma" color="black" size="1"><a name="ref3">3.</a> Marco Del Corso, Michael Toffler, David M Dohan Ehrenfest. Use of an autologous leukocyte and platelet-rich fibrin (L-PRF) membrane in post avulsion sites: An overview of Choukroun’s PRF. Jo Im Adv Clin Dent 2010; 1(9):27-35.</font></p> | + | <p class="ref-label"><font face="Arial" color="black" size="1"><a name="ref3">3.</a> Marco Del Corso, Michael Toffler, David M Dohan Ehrenfest. Use of an autologous leukocyte and platelet-rich fibrin (L-PRF) membrane in post avulsion sites: An overview of Choukroun’s PRF. Jo Im Adv Clin Dent 2010; 1(9):27-35.</font></p> |
| − | <p class="ref-label"><font face="Tahoma" color="black" size="1"><a name="ref4">4.</a> Ziv Mazor, Robert A. Horowitz, Marco Del Corso, Hari S Prasad, Michael D Rohrer, and David M Dohan Ehrenfesti. Sinus floor augmentation with simultaneous implant placement using choukroun’s platelet-rich fibrins the sole grafting material: a radiologic and histologicstudy at 6 months. J Periodontol 2009; 80(12):2056-64.</font></p> | + | <p class="ref-label"><font face="Arial" color="black" size="1"><a name="ref4">4.</a> Ziv Mazor, Robert A. Horowitz, Marco Del Corso, Hari S Prasad, Michael D Rohrer, and David M Dohan Ehrenfesti. Sinus floor augmentation with simultaneous implant placement using choukroun’s platelet-rich fibrins the sole grafting material: a radiologic and histologicstudy at 6 months. J Periodontol 2009; 80(12):2056-64.</font></p> |
| − | <p class="ref-label"><font face="Tahoma" color="black" size="1"><a name="ref5">5.</a> Joseph Choukroun, Antoine Diss, Alain Simonpieri et.al. Platelet-rich fibrin (PRF): A second-generation platelet concentrate.Part IV: Clinical effects on tissue healing. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2006; 101(3):56-60.</font></p> | + | <p class="ref-label"><font face="Arial" color="black" size="1"><a name="ref5">5.</a> Joseph Choukroun, Antoine Diss, Alain Simonpieri et.al. Platelet-rich fibrin (PRF): A second-generation platelet concentrate.Part IV: Clinical effects on tissue healing. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2006; 101(3):56-60.</font></p> |
| − | <p class="ref-label"><font face="Tahoma" color="black" size="1"><a name="ref6">6.</a> Cawood JI, Howell RA. A Classification of the Edentulous Jaws. Int J Oral Maxillofac Surg 1988; 17(4):232-6.</font></p> | + | <p class="ref-label"><font face="Arial" color="black" size="1"><a name="ref6">6.</a> Cawood JI, Howell RA. A Classification of the Edentulous Jaws. Int J Oral Maxillofac Surg 1988; 17(4):232-6.</font></p> |
| − | <p class="ref-label"><font face="Tahoma" color="black" size="1"><a name="ref7">7.</a> Jensen OT et al. Report of the sinus consensus conference of 1996. Int J Oral Maxillofac Implants 1998; 13:11-45.</font></p> | + | <p class="ref-label"><font face="Arial" color="black" size="1"><a name="ref7">7.</a> Jensen OT et al. Report of the sinus consensus conference of 1996. Int J Oral Maxillofac Implants 1998; 13:11-45.</font></p> |
| − | <p class="ref-label"><font face="Tahoma" color="black" size="1"><a name="ref8">8.</a> Bjorn Johansson, Karin Wannfors, Jan Ekenback, Jan-Ivan Smedberg, Jan Hirsch. Implants and Sinus-Inlay Bone Grafts in a 1-Stage Procedure on Severely Atrophied Maxillae: Surgical Aspects of a 3-Year Follow-up Study. Int J Oral Maxillofac Implants 1999; 14(6):811-8.</font></p> | + | <p class="ref-label"><font face="Arial" color="black" size="1"><a name="ref8">8.</a> Bjorn Johansson, Karin Wannfors, Jan Ekenback, Jan-Ivan Smedberg, Jan Hirsch. Implants and Sinus-Inlay Bone Grafts in a 1-Stage Procedure on Severely Atrophied Maxillae: Surgical Aspects of a 3-Year Follow-up Study. Int J Oral Maxillofac Implants 1999; 14(6):811-8.</font></p> |
| − | <p class="ref-label"><font face="Tahoma" color="black" size="1"><a name="ref9">9.</a> Peleg M, Mazor Z, Garg AK. Augmentation grafting ofthe maxillary sinus and simultaneous implant placementin patients with 3 to 5 mm of residual alveolarbone height. Int J Oral Maxillofac Implants 1999; 14(4):549-56.</font></p> | + | <p class="ref-label"><font face="Arial" color="black" size="1"><a name="ref9">9.</a> Peleg M, Mazor Z, Garg AK. Augmentation grafting ofthe maxillary sinus and simultaneous implant placementin patients with 3 to 5 mm of residual alveolarbone height. Int J Oral Maxillofac Implants 1999; 14(4):549-56.</font></p> |
| − | <p class="ref-label"><font face="Tahoma" color="black" size="1"><a name="ref10">10.</a> Gunther KP, Scharf HP, Pesch HJ, Puhl W. Osteointegration of solvent preserved bone transplants in an animal model. Osteologie 1996; 5:4-12.</font></p> | + | <p class="ref-label"><font face="Arial" color="black" size="1"><a name="ref10">10.</a> Gunther KP, Scharf HP, Pesch HJ, Puhl W. Osteointegration of solvent preserved bone transplants in an animal model. Osteologie 1996; 5:4-12.</font></p> |
| − | <p class="ref-label"><font face="Tahoma" color="black" size="1"><a name="ref11">11.</a> Sung Han Sul, Byung Ho Choi, Jingxu Li, Seung MiJeong, and Feng Xuan. Effects of sinus membrane elevation on bone formation around implants placed in the maxillary sinus cavity: an experimental study. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2008; 105(6):684-7.</font></p> | + | <p class="ref-label"><font face="Arial" color="black" size="1"><a name="ref11">11.</a> Sung Han Sul, Byung Ho Choi, Jingxu Li, Seung MiJeong, and Feng Xuan. Effects of sinus membrane elevation on bone formation around implants placed in the maxillary sinus cavity: an experimental study. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2008; 105(6):684-7.</font></p> |
| − | <p class="ref-label"><font face="Tahoma" color="black" size="1"><a name="ref12">12.</a> Bin Shi, Yi Zhou, Yi Ning Wang, Xiang Rong Cheng. Alveolar ridge preservation prior to implant placement with surgical- grade calcium sulphate and platelet rich plasma: A pilot study in a canine model. Int. J Oral Maxillofac Implants 2007; 22(4):656-65.</font></p> | + | <p class="ref-label"><font face="Arial" color="black" size="1"><a name="ref12">12.</a> Bin Shi, Yi Zhou, Yi Ning Wang, Xiang Rong Cheng. Alveolar ridge preservation prior to implant placement with surgical- grade calcium sulphate and platelet rich plasma: A pilot study in a canine model. Int. J Oral Maxillofac Implants 2007; 22(4):656-65.</font></p> |
| − | <p class="ref-label"><font face="Tahoma" color="black" size="1"><a name="ref13">13.</a> Ling He, Ye Lin, Xiulian Hu, Yu Zhang, and Hui Wu. A comparative study of platelet-rich fibrin (PRF) and platelet- rich plasma (PRP) on the effect of proliferation and differentiation of rat osteoblasts in vitro. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2009; 108(5):707.</font></p></div> | + | <p class="ref-label"><font face="Arial" color="black" size="1"><a name="ref13">13.</a> Ling He, Ye Lin, Xiulian Hu, Yu Zhang, and Hui Wu. A comparative study of platelet-rich fibrin (PRF) and platelet- rich plasma (PRP) on the effect of proliferation and differentiation of rat osteoblasts in vitro. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2009; 108(5):707.</font></p></div> |
| | </div> | | </div> |
| | </div> | | </div> |